
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
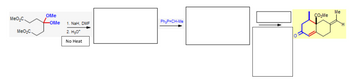
Transcribed Image Text:MeO₂C.
MeO₂C.
OMe
-OMe
1. NaH, DMF
2. H₂O*
No Heat
Ph₂P=CH-Me
CO₂Me
D
Me
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Specific heat of Ag = 0.240 J/g°C, Al = 0.921 J/g°C, Pb = 0.138 J/g°C %3D %3D 1. If a piece of aluminum with a mass of 3.99 g and a temperature of 100.0 °C is dropped in 10.0 cm of water at 21.0 °C, what will be the final temperature of the system? (Hint: Remember the density of water is 1.00 g / mL, 'c' for Al is 0.921 J/g*°C) (Ans:27.40C) CH3CH=CH2 3.arrow_forwardd. Calculate the amount of heat released when 1.26 x 101 g of NO2 are produced according to the following chemical equation. (5 marks) Hitung jumlah haba yang dibebaskan apabila 1.26 x 10 g NO2 dihasilkan mengikut persamaan kimia berikut. (5 markah) 2NO(g) + 02(g) → 2NO2(g) AH= -114.6 kJmol1arrow_forward11. A combustion reaction releases 1.435 x 10° kJ of heat that is absorbed by 8.00 kL of 22.1 °C water. -1 What is the final temperature (in °C) of the water? (Cs water =4-180J g°C; dwater = 1.00 g mL-) %3D 4.184arrow_forward
- Cl2 (g) + H2 (g) → 2 HCl (g) ΔH0rxn = ‒184.6 kJ 6. The reaction is ___________ as it _____________ heat. Group of answer choices A.exothermic, absorbs B.exothermic, releases endothermic, C.releases endothermic, absorbs D.Not enough information to determinearrow_forwardAn unknown organic compound is being proposed as an alternative to gasoline. The enthalpy of combustion of this compound is 292kcal mol1. The density is 0.258g mL1. The molecular weight of this compound is 362g mol1. How much heat in kcal is released from combustion of 0.28 L of organic compound? Round your answer to 2 decimal places.arrow_forwardPredict the reagent(s) needed to produce this regiochemistry in this elimination reaction. •·||| Q A B C D H3O+ heat CH3OH CH3OH heat CH3ONa heat ( Donearrow_forward
- 6.D Macmillan Question 10 of 13 > 9= Calculate the heat of solution for KOH in kJ/mol. heat of solution = A chemist dissolved an 11.9-g sample of KOH in 100.0 grams of water in a coffee cup calorimeter. When she did so, the wat temperature increased by 26.0 °C. Based on this, how much heat energy was required to dissolve the sample of KOH? Assun the specific heat of the solution is 4.184 J/g °C. E OO Resources Lx Give Up? a HUAWEI P30 LEICA TRIPLE CAMERA Hint Check Answer Attempt 3 11:06 PM 11/21/2022 kJ/arrow_forwardIN B C. LI %#3 Assume that it is possible to heat water with perfect insulation (all the heat from combustion of ethanol is transferred to water). How much energy (in joules) is needed to heat 200.0 g of water by 10.0 °C? The heat capacity of water is 4.185 J/g · °C. energy: How many millimoles of ethanol would need to be burned to generate the amount of energy that is needed to heat 200.0 g of water by 10.0 °C? The heat of combustion of ethanol is –1368 kJ/mol. millimoles of ethanol: mmol What is the volume of ethanol required to heat 200.0 g of water by 10.0 °C? The density of ethanol is 0.78 g/mL. volume: MacBook Pro Q Search or enter website name 田) & %24 delete 4. 7. 5. R. P. D H. K. M alt gE MOSISO command optionarrow_forwardDetermine the amount of heat (in kJ) given off when 1.86 x 10° g of NO2 are produced according to the equation: 2NO(g) + O,(g) → 2NO2(g) AH = -114.6 kJ/mol (N=14, O=16) 2.32 x 10' kj 4.64 x 10' kj 2.32 x 10* kj 1.16 x 10° kj 4.64 x 10° kjarrow_forward
- 2 SO2 (g) + 2 H2O (1) → 2 H,S (g) +3 O2 (g) AHren 1124 kJ mol a. Is this reaction exothermic or endothermic? O endothermic O exothermic b. How many moles of gaseous SO2 must react if 2.46 kJ of heat are absorbed? number or fraction only molarrow_forwardWhich of the reaction below is an exothermic process and a combustion reaction? a. Al2O3(s) + 3 C (graphite -> 2Al(s) + 3 CO (G) ΔH= 1344.5kJ/mol b. S(s) + (g) -> SO2(g) ΔH = -296.8 kJ/mol c. PH(3) + 2O2(g) -> H3PO4 Δ H= -12867.4 d.) C4H10(g 13/2O29(g) -> 4CO2(g) + 5H20 ΔH= -890 kJ/molarrow_forward1. What is energy? What are the various units that energy is measured in?2. What do the variables in ? = ??Δ? mean?3. What does Δ? = ?? − ?? mean? What is the meaning of a negative Δ??4. 20 grams of water (heat capacity = 4.184 J/(gOC)) increases in temperature by 15OC. How much heat energy was required?5. What is a coffee cup calorimeter? Why do we want such a calorimeter to beinsulated?6. Explain how temperature change with the same amount of energy supplied will varydepending on the heat capacity of a substance.7. What is an exothermic reaction? An endothermic reaction?8. What happens with energy when a chemical bond forms? When a bond breaks?9. What is Δ? called? What does it mean if Δ? is positive? Negative?10.What is Hess’s Law?11. If ? + ? → ??, Δ? = −100 ?? and ?? → ? + ?, Δ? = +50 ??, then find Δ? for:? + ?? → ? + ??, Δ? = ? ?12.What is the relationship between frequency and wavelength of light?13. Identify all variables in ? = ℎ? and ? =??14.Calculate the frequency of 450 nm…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY