
Biology 2e
2nd Edition
ISBN: 9781947172517
Author: Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Question
Please step by step answer and don't use ASI Answer please
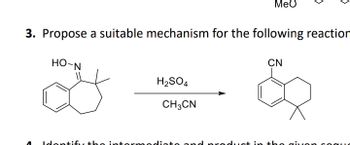
Transcribed Image Text:MeO
3. Propose a suitable mechanism for the following reaction
HO-N
interm
H2SO4
CH3CN
in
CN
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- In an enzymatic reaction: a. the enzyme leaves the reaction chemically unchanged. b. if the enzyme molecules approach maximal rate, and the substrate is continually increased, the rate of the reaction does not reach saturation. c. in the stomach, enzymes would have an optimal activity at a neutral pH. d. increasing temperature above the optimal value slows the reaction rate. e. the least important level of organization for an enzyme is its tertiary structure.arrow_forwardWhich of the following statements about the allosteric site is true? a. The allosteric site is a second active site on a substrate in a metabolic pathway. b. The allosteric site on an enzyme can allow the product of a metabolic pathway to inhibit that enzyme and stop the pathway. c. When the allosteric site of an enzyme is occupied, the reaction is irreversible and the enzyme cannot react again. d. An allosteric activator prevents binding at the active site. e. An enzyme that possesses allosteric sites does not possess an active site.arrow_forwardEnergetic of Fructose-1 ,6-bis P Hydrolysis (Integrates with Chapter 3.) The standard free energy change (G) for hydrolysis of fructose-1. 6-bisphosphate (FBP) to fructose-S-phosphate (F-6-P) and P: is -16.7 KJ/mol: FBP + H2O fructose-6-P + Pi The standard free energy change (G) for ATP hydrolysis is -30.5 KJ/mol: ATP + H2O ADP + Pj What is the standard free energy change for the phosphofructokinase reaction: ATP + fructose-6-P ADP + FBP b. What is the equilibrium constant for this reaction? c. Assuming the intracellular concentrations of [ATP] and (ADP] are maintained constant at 4 mM and 1.6 mM, respectively, in a rat liver cell, what will be the ratio of [FBP]/[fructose-6-P] when the phosphofructokinase reaction reaches equilibrium?arrow_forward
- Which of the following statements about inhibition is true? a. Allosteric inhibitors and allosteric activators are competitive for a given enzyme. b. If an inhibitor binds the active site, it is considered noncompetitive. c. If an inhibitor binds to a site other than the active site, this competitive inhibition. d. A noncompetitive inhibitor is believed to change the shape of the enzyme, making its active site inoperable. e. Competitive inhibition is usually not reversible.arrow_forwardUnderstanding the Mechanisms of Reactions Related to Transketolase The mechanistic chemistry of the acetolactate synthase and phosphoketolase reactions (shown here) is similar to that of the transketolase reaction (Figure 22.30). Write suitable mechanisms for these reactions.arrow_forwardWhich of the following is the best way to judge the relative activation energies between two given chemical reactions? Compare the ?G values between the two reactions Compare their reaction rates Compare their ideal environmental conditions Compare the spontaneity between the two reactions.arrow_forward
- Using the ActiveModel for phosphofructokinase (Trypanosoma), describe the difference between the APO1, AP02, and holoenzyme conformations.arrow_forwardDescribe the position of the transition state on a vertical energy scale, from low to high, relative to the position of the reactants and products, for both endergonic and exergonic reactions.arrow_forwardUsing the ActiveModel for aldose reductase, describe the structure of the TIM barrel motif and the structure and location of the active site.arrow_forward
- Examine the ActiveModel for alcohol dehydrogenase and describe the structure and function of the catalytic zinc center.arrow_forwardWhy Do Anabolic and Catabolic Pathways Differ? Why is the pathway for the biosynthesis of a biomolecule at least partially different from the pathway for its catabolism? Why is the pathway for the biosynthesis of a biomolecule inherently more complex than the pathway for its degradation?arrow_forwardFigure 27.3 illustrates the response of R (ATP-regenerating) and U (ATP-utilizing) enzymes to energy charge. a. Would hexokinase be an R enzyme or a U enzyme? Would glutamine: PRPP amidotransferase, the second enzyme in purine biosynthesis, be an R enzyme or a U enzyme? b. If energy charge = 0.5: Is the activity of hexokinase high or low? Is ribose-5-P pyrophosphokinase activity high or low? c. If energy charge = 0.95: Is the activity of hexokinase high or low? Is ribose-5-P pyrophosphokinase activity high or low?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781337408332Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781337408332Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781305073951Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781305073951Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Biology: The Unity and Diversity of Life (MindTap...
Biology
ISBN:9781337408332
Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Biology: The Unity and Diversity of Life (MindTap...
Biology
ISBN:9781305073951
Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Anatomy & Physiology
Biology
ISBN:9781938168130
Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:OpenStax College