
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
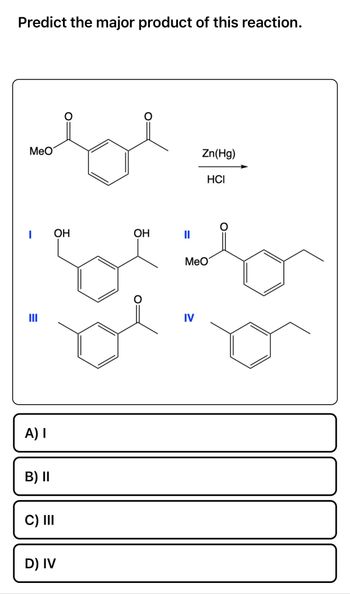
Transcribed Image Text:Predict the major product of this reaction.
MeO
A) I
B) II
C) III
OH
D) IV
OH
||
Zn(Hg)
IV
HCI
MeO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can anyone please help me to solve this problem?I am stuck please help ( This question is not a part of any graded assignment) So please help me.arrow_forwardGive detailed Solution with explanationarrow_forward$34000 SOS Br O 1.mCPBA 2.H*, MeOH 3. PCC 2 (RTM2 av elorlog to raitons 8-99 1.Mgº in ether 2. Q 2 3. PCC OTISP 1.MeLi then H3O+ OH 1.TSCI, pyridine 2. LIAIH4 Br OH terito O.N nont 1.Br2, hv 2. LiOH [nuc.] 3. H₂CRO4 19 T HO HOSM HO i zalog 1.NaBH4 2.Pl3 OH O 3. OH 1.MeLi then H3O+ LOTS 1.NaOH (base) 2. HCI, ROOR MgBr 1. 1.PhMgBr 2. POCI3, pyridine 3. CH₂N2, hv OTIPS MAJOT enibinya Li 2. TBAF then H3O+ Name the following alcoholarrow_forward
- Nonearrow_forwardWOD ni mi 916 Doy leve 11 An aqueous solution has A hydroxine lon Concentration of 1.0 x 10-7 m. M.00 What is the hgc He word) hip nila mwa (noleinword) reeg exil jaut nila st Solution? uoda omized SAT Concentration= т moy ebou even bi Is this solution acidic, basic, veneute 152,323 ybod ion concentration in this onids Orighe,szle ybodyns sal way abril 19arrow_forwardE' I с E 1. Phenylamine is an aromatic amine that is used in the manufacture of dyes. When absorbed through the skin it causes the Fe+2 in hemoglobin to become oxidized into Fe+3, resulting in the formation of methemoglobin which cannot bind to or transport oxygen. Phenylamine is soluble in water and acts as a weak base. a. a. CøHşNHz (aq) + H2O (l) = CHşNH;* (aq) + OH (aq) When you measure the concentrations of the ionized substances you find them to be: [C6H5NH₂] = 0.234 mol/L [C6H5NH3*] = 2.8 x 10³ mol/L [OH-]= 2.8 x 10³ mol/L If the K is 4.27 x 10-10, is the reaction at equilibrium? If not, which direction does it need to move (right or left) to reach equilibrium? Explain. At equilibrium the concentrations of the ionized substances are: [C6H5NH₂] = 0.0537 mol/L [C6H5NH3*] =4.79 x 10€ mol/L [OH-]= 4.79 x 10¹ mol/L If this reaction is taking place in a 2.0L container, and 1.5 moles of phenylamine were added to the reaction, what will the new concentrations of the three ionic species…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY