
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
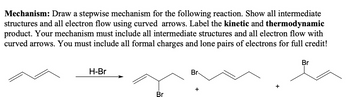
Transcribed Image Text:Mechanism: Draw a stepwise mechanism for the following reaction. Show all intermediate
structures and all electron flow using curved arrows. Label the kinetic and thermodynamic
product. Your mechanism must include all intermediate structures and all electron flow with
curved arrows. You must include all formal charges and lone pairs of electrons for full credit!
Br
H-Br
Br
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Substitution and elimination: predict the product Maximum allowed tries per question: Unlimited (7) Draw the major organic product of the reaction. Follow this procedure: determine whether the reaction conditions are acidic or basic; identify the most nucleophilic/basic atom, the electrophilic atom, and the leaving group; predict whether elimination or substitution will occur; and then draw the product. Indicate the stereochemistry at every stereocenter with a single wedged (up), hashed (down), or wavy (a mixture of up and down; either) bond. Launch MarvinJSTM viewer or click image to copy source CH3 H3C EtONa EtOH Brarrow_forwardCan I get help with thisarrow_forwardDraw a step-by-step mechanism for the transformation shown below (no additional reagents are needed), showing all electron flow with arrows.arrow_forward
- 5. Using curved arrows show the mechanism for the hydrohalogenation for the following reaction. Show how the two possible intermediate products are formed and explain why only one product proceeds to form the product. Clearly indicate attack of electrons and full and partial (8) charges. H-Br Br H-Br Product Intermediate Productsarrow_forward2- Mention the product of a nucleophilic substitution reaction of (S)-2-bromohexane with acetate ion, CH3CO2- Assume that inversion of configuration occurs, and show the chemistry of both the reactant and product. 3- Draw the mechanism of the SN2 Reaction. Show the correct directions of the arrows, and all reagents. 4- Draw the mechanism of the SN1 Reaction. Show the correct directions of the arrows, and all reagents. 5- Draw the mechanism of the E2 Reaction with an Alkyl Halide. Show the correct directions of the arrows, and all reagents. 6- Draw the mechanism of the E1 Reaction with an Alkyl Halide. Show the correct directions of the arrows, and all reagents. 7- Mention the product formed in an SN2 reaction between 1-bromobutane and NaI. 8- Rank the following compounds in order of their expected reactivity toward SN2 reaction: CH3Br, CH3OTos, (CH3)2CHCl. 9- Explain Grignard Reagents in details with one example. 10- Mention how a halogen substituent can be replaced by a deuterium atom…arrow_forwardHelp me draw in the arrows, thank you.arrow_forward
- 4. Use the reactant below to perform two separate reactions. Give the mechanism(s) and product(s) for each reaction. Show stereochemistry and be clear your work. H₂SO4, EtOH D !!!H Me NaOEt, EtOHarrow_forward12B. CARBONYL REACTION MECHANISMS Draw the arrow-pushing mechanism for the reactions, including all charged intermediates and product. Mix & Match (Mechanism Bootcamp): draw the mechanism for each starting material (1-6) and reagent from the 12B reactions (previous page) (1a) NABH4 MeOHarrow_forwardI need help with this question pleasearrow_forward
- Draw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. CH3 CH3 H3PO4 HO, + H3C CH3 HO HO First stage in synthesis of the epoxy and polycarbonate ingredient bisphenol-A You do not have to consider stereochemistry. Include all valence lone pairs in your answer. In cases where there is more than one answer, just draw one.arrow_forward8. Draw a plausible multi-step mechanism for the following transformation. Remember to use the correct arrow conventions for indicating electron flow and to keep track of relevant lone pairs and formal charges. H₂C O CH₂ H -OH H-O-H H H₂C O CH3arrow_forward5а. ( Show an SN2 mechanism for the following reaction. Include the transition state, with stereochemistry. Use your work to predict the product, including stereochemistry. Br H acetone 5b. predict the product, including stereochemistry. Show an SN1 mechanism for the following reaction. Use your work to HOʻ Br Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY