
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
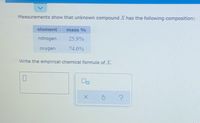
Transcribed Image Text:Measurements show that unknown compound X has the following composition:
element
mass %
nitrogen
25.9%
oxygen
74.0%
Write the empirical chemical formula of X.
Expert Solution
arrow_forward
Step 1
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Arsenic reacts with oxygen to form a compound AsxOy. If 2.354g of arsenic forms 3.611g of the compound form. What is the empirical formula for the compound?arrow_forwardDetermine the empirical formula for a compound whose percent composition is 53.90% C, 10.20% H, and 35.90% O.arrow_forwardHow many formula units make up 35.8 gg of magnesium chloride (MgCl2MgCl2arrow_forward
- 5) A certain compound has the following percent composition: 57.1% C, 4.8% H, and 38.1 % O. If the molar mass of this compound is 126 g/mol, what is the molecular formula?arrow_forwardthe analysis of a compound shows that it contains 5.4 mol c, 7.2 mol h, and 1.8 mol n. what is the empirical formula of the compound?arrow_forwardA compound used in the nuclear industry has the following composition: uranium, 67.61% ; fluorine, 32.39% . Determine the empirical formula of the compound.arrow_forward
- There is a series of nitrogen oxides that have the general formula NxOy. What is the empirical formula of the nitrogen oxide compound that contains 25.94% nitrogen by mass?arrow_forwardA common salt has the following formula: XCl2◦6H2O. The compound is 29.8% chlorine, 40.4% oxygen and 5.1% hydrogen. Determine the molar mass of the compound and identify the element X.arrow_forwardplease helparrow_forward
- Determine the number of moles (of molecules or formula units) in sample. 0.1187 g C8H18arrow_forwardConvert mass % to grams (assume 100gexactly)•Convert grams to moles.•Write simplest ratio of the elements. (Usually works by dividing the smallest number into the other numbers)•Write a formula with the closest small whole numbers from the ratio.arrow_forwardWhich one of these ions has the largest radiust Select one: a. Ci 2- b. s + с. К + d. Na 2- е. Оarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY