
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Пру
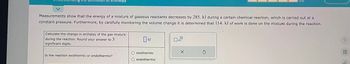
Measurements show that the energy of a mixture of gaseous reactants decreases by 285. kJ during a certain chemical reaction, which is carried out at a
constant pressure. Furthermore, by carefully monitoring the volume change it is determined that 114. kJ of work is done on the mixture during the reaction.
Calculate the change in enthalpy of the gas mixture
during the reaction. Round your answer to 3
significant digits.
Is the reaction exothermic or endothermic?
kJ
exothermic
endothermic
x10
X
0/5
Ś
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A calorimeter holds 55 g water at 21.0°C. A sample of hot iron is added to the water. The final temperature of the water and iron is 24.0°C. What is the change in enthalpy associated with the change in the water's temperature?arrow_forwardMeasurements show that the energy of a mixture of gaseous reactants decreases by 206. kJ during a certain chemical reaction, which is carried out at a constant pressure. Furthermore, by carefully monitoring the volume change it is determined that -145. kJ of work is done on the mixture during the reaction. Calculate the change in enthalpy of the gas mixture during the reaction. Be sure your answer has the correct number of significant digits. O exothermic Is the reaction exothermic or endothermic? O endothermicarrow_forwardThe work done to compress a gas is 70 J. As a result, 25 J of heat is given off to the surroundings. Calculate the change in energy of the gas.arrow_forward
- A 1.37 g sample of butane, C4H10(g), was burned in a bomb calorimeter in the presence of excess oxygen. The calorimeter contained 1.09 kg of water. Calculate the heat exchanged with respect to the system during the reaction if the calorimeter has a heat capacity of 648. J/°C and the temperature of the calorimeter and water changed from 23.6°C to 35.6°C? Enter your answer to 3 significant figures in decimal form. Do not enter units since they are already given. 58.12 kJ Calculate the heat exchanged with respect to the system per mole of butane. Enter your answer to 3 signficant figures in decimal form. Do not enter units since they are already given.’ 2547.5 kJarrow_forwardMeasurements show that the energy of a mixture of gaseous reactants increases by 396. kJ during a certain chemical reaction, which is carried out at a constant pressure. Furthermore, by carefully monitoring the volume change it is determined that 122. kJ of work is done on the mixture during the reaction. Calculate the change in enthalpy of the gas mixture during the reaction. Be sure your answer has the correct number of significant digits. O exothermic Is the reaction exothermic or endothermic? O endothermicarrow_forwardMeasurements show that the enthalpy of a mixture of gaseous reactants decreases by 323. kJ during a certain chemical reaction, which is carried out at a constant pressure. Furthermore, by carefully monitoring the volume change it is determined that -162. kJ of work is done on the mixture during the reaction. Calculate the change in energy of the gas mixture during the reaction. Round your answer to 3 significant digits. Is the reaction exothermic or endothermic? kJ exothermic O endothermic x10 X 3arrow_forward
- A 55.1g sample of polystyrene, which has a specific heat capacity of 1.880·J·g−1°C−1, is put into a calorimeter (see sketch at right) that contains 200.0g of water. The temperature of the water starts off at 25.0°C. When the temperature of the water stops changing it's 31.9°C. The pressure remains constant at 1atm . Calculate the initial temperature of the polystyrene sample. Be sure your answer is rounded to the correct number of significant digits.arrow_forwardAs a system increases in volume, it absorbs 52.0 J of energy in the form of heat from the surroundings. The piston is working against a pressure of 0.597 atm. The final volume of the system is 58.6 L. What was the initial volume of the system if the internal energy of the system decreased by 106.2 J? Volume = Larrow_forwardA chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 4.70 kg of water at 38.7 °C. During the reaction 55.6 kJ of heat flows out of the flask and into the bath. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is -1 4.18 J∙g¯¹·K¯¹. Be sure your answer has the correct number of significant digits. 心。 °℃ x10 Sarrow_forward
- Measurements show that the energy of a mixture of gaseous reactants decreases by 373. kJ during a certain chemical reaction, which is carried out at a constant pressure. Furthermore, by carefully monitoring the volume change it is determined that 136. kJ of work is done on the mixture during the reaction. Calculate the change in enthalpy of the gas mixture during the reaction. Round your answer to 3 x10 significant digits. exothermic Is the reaction exothermic or endothermic? endothermicarrow_forwardMeasurements show that the enthalpy of a mixture of gaseous reactants increases by 195.kJ during a certain chemical reaction, which is carried out at a constant pressure. Furthermore, by carefully monitoring the volume change it is determined that 69.kJ of work is done on the mixture during the reaction. Calculate the change in energy of the gas mixture during the reaction. Be sure your answer has the correct number of significant digits. Is the reaction exothermic or endothermic?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY