
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Match the items please.
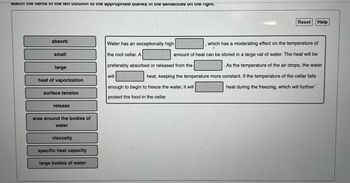
Transcribed Image Text:Match the items in the left column to the appropriate Dianks in the sentences on the right.
absorb
small
large
heat of vaporization
surface tension
release
area around the bodies of
water
viscosity
specific heat capacity
large bodies of water
Water has an exceptionally high
the root cellar. A
preferably absorbed or released from the
will
Reset Help
which has a moderating effect on the temperature of
amount of heat can be stored in a large vat of water. The heat will be
As the temperature of the air drops, the water
heat, keeping the temperature more constant. If the temperature of the cellar falls
heat during the freezing, which will further
enough to begin to freeze the water, it will
protect the food in the cellar.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the volume measurement indictatede in the volumetric glassware ? 1. Graduated Cylinder (PINK) 2. Delivery Pipettearrow_forwardгрчно чуд ч зыри: 7'чні | |Iarrow_forward(Handwritten answer) Calculate range of volumes representing 0.1% tolerance of a 25.00 mL volumetric pipette assuming that 25.00 mL is the dispensed volume!arrow_forward
- What piece of glassware was missing from the simple distillation setup? Group of answer choices Condensor Vacuum adaptor Thermometer adaptor Three-way adaptorarrow_forward2140.56+9.3456arrow_forwardIn lab, you add 1.985 g salicylic acid to a 125 mL Erlenmeyer flask, as listed in the procedure. You carefully add 4.0 mL acetic anhydride, swirl for a few minutes, then have your TA add 5 drops of sulfuric acid. After completing the synthesis and filtering, you place the crystals to dry on an evaporating dish that weights 21.173 g. After drying, the crystals and evaporating dish weigh 23.472. Calculate the percent yield of aspirin. Insert the value as a percentage, but without the percent sign.arrow_forward
- S1: Revisit Luisa could you change L "small particles" more accurate Ultrascope views? 52: Examine the following 10,000,000 x magnification Ultrascope views. Indicate whether each view represents an element, compound, or mixture/solution. Model A Model B 10.000 000 x Model C 10.000.000 10,000,000 x TRASCOPE ULTRASCOPE ULTRASCOPE "I'm confused! Water is ndarrow_forward6. Which of the following is consistent with the 'least to most accurate' glassware for measuring 10 mL? 25 mL beaker, 25 mL graduated cylinder, 10 mL graduated pipet, 10 mL volumetric pipet 25 mL Erlenmeyer flask, 10 mL graduated pipet, 10 mL volumetric pipet, 25 mL graduated cylinder 10 mL volumetric pipet, 10 mL graduated pipet, 25 mL graduated cylinder, 25 mL beaker O 10 mL graduated pipet, 10 mL volumetric pipet, 25 mL beaker, 25 mL graduated cylinderarrow_forwardDensity of lead 75.63g (4 rods), start 21.0 ml, end 16.80mlarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY