
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
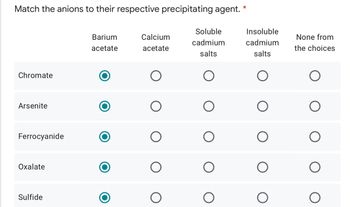
Transcribed Image Text:Match the anions to their respective precipitating agent.
Chromate
Arsenite
Ferrocyanide
Oxalate
Sulfide
Barium
acetate
Calcium
acetate
O
O
O
Soluble
cadmium
salts
O
O
O
*
Insoluble
cadmium
salts
O
None from
the choices
O
O
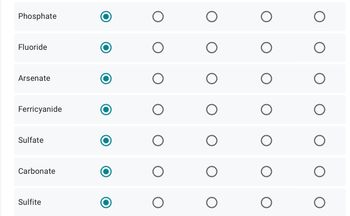
Transcribed Image Text:Phosphate
Fluoride
Arsenate
Ferricyanide
Sulfate
Carbonate
Sulfite
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Write the balanced net ionic equation, including the phases, for the given reaction. A solution of Ba(OH), and a solution of H,S0, are mixed. Water and a precipitate of BaSO . balanced net ionic equation:arrow_forwardHand written solution is not allowed and no answer from the chat gpt will dislikearrow_forwardAqueous solutions of 0.1 M copper (II) chloride and 0.1 M silver nitrate are combined and allowed to react. From the following, select all of the statements that are true. A The product of the reaction will have a colored supernatant B The product of the reaction will have a colorless supernatant C The reaction will produce a gas D The reaction will produce a precipitate E The reaction will produce water as a product F The reaction will result in an oxidation and/or reduction of one of the reactantsarrow_forward
- Which of the following will occur when an aqueous solution of KBr is mixed with an aqueous solution of AGNO3? No precipitate will form. OA Aprecipitate of KNO3 will form. OB Oc A precipitate of AgBr will form. OD A precipitate of AgNo3 will form. DE Bright yellow precipitate will form.arrow_forward12. In a titration of HNO3, you add a few drops of phenolphthalein indicator to 50.00 L if acid in a flask. You quickly add 20.00 mL of 0.0502 M NaOH but overshoot the endpoint, turning the solution a deep pink color. Instead of starting over you add an additional 30.00 mL of the acid, turning the solution colorless. It then takes 3.22 mL of the NaOH to reach the endpoint. a. Write the Molecular, Total Ionic, and Net Ionic Equations for this reaction. b. What is the concentration of the HNO3 solution? c. How many moles of NaOH were in excess after the first addition?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY