
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:3.3-3.4
Do you wa
updates n
2 3 4 5 6 7
1
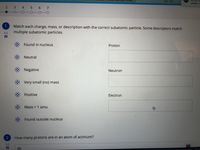
Match each charge, mass, or description with the correct subatomic particle. Some descriptors match
4.5
multiple subatomic particles.
Found in nucleus
Proton
Neutral
Negative
Neutron
Very small (no) mass
Positive
Electron
Mass = 1 amu
Found outside nucleus
How many protons are in an atom of actinium?
0.5
89
Expert Solution
arrow_forward
Step 1
The atom has three sub-atomic particles namely electron, proton and neutron.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill in the blanks in the table below about an atom’s subatomic particles. Subatomic Particle Location Charge Weight proton electron cloud 0 AMU neutralarrow_forwardComplete the table below, using the diagram of an atom shown at right. name symbol 0 0 0 n Properties of subatomic particles approximate mass (amu) charge (in multiples of e) -1 0 0 0.0005 (choose one) 1.0 location on diagram B (choose one) A X 5arrow_forwardEnter your answer in the provided box. 121 The atomic masses of the two stable isotopes of antimony, Sb (57.30 percent) and 51 123 Sb (42.70 percent), are 120.9038 amu and 122.9042 amu, respectively. 51 Calculate the average atomic mass of antimony. amuarrow_forward
- Isotopes are elements of the same type with different numbers of resulting in differing . protons/masses O neutrons/charge neutrons/masses electrons/massesarrow_forwardThere are three primary subatomic particles that comprise an atom. (i) Name the three subatomic particles, (ii)state where each particle is located in an atom, and (iii) state the charge of each particle. You made include a diagram as part of your answer.arrow_forwardUse the dropdowns to create a correct and complete isotope notation. An isotope has 108 neutrons and 72 protons. The correct isotope is:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY