
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
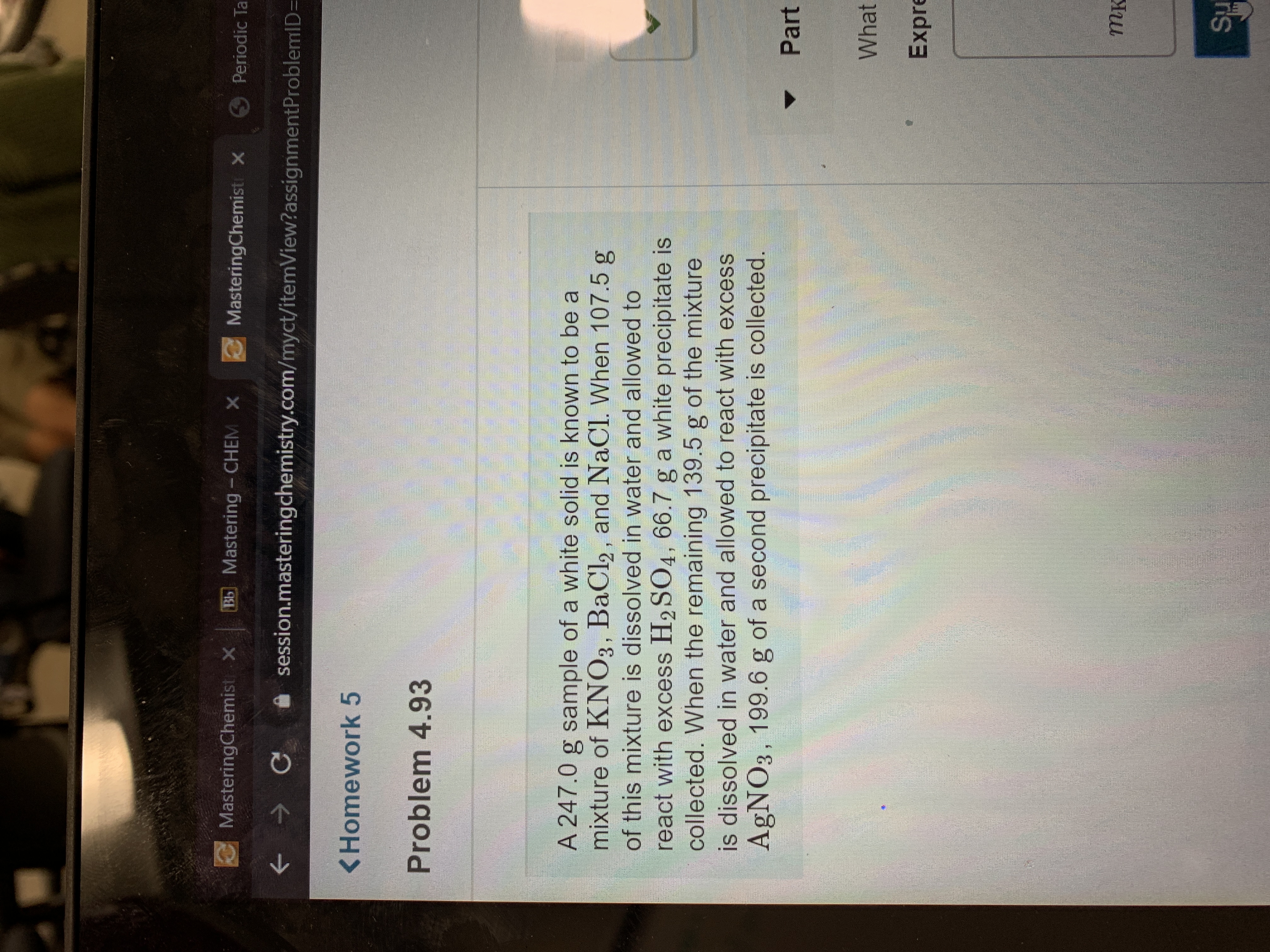
What is the mass of KNO3 in the original 247g mixture of KNO3 BaCl2 and NaCl
Transcribed Image Text:MasteringChemist X
Bb Mastering- CHEM X
MasteringChemist x
S Periodic Ta
C
session.masteringchemistry.com/myct/itemView? assignmentProblemID=
<Homework 5
Problem 4.93
A 247.0 g sample of a white solid is known to be a
mixture of KNO3, BaCl2, and NaCl. When 107.5 g
of this mixture is dissolved in water and allowed to
react with excess H2 SO4, 66.7 g a white precipitate is
collected. When the remaining 139.5 g of the mixture
is dissolved in water and allowed to react with excess
AgNO3, 199.6 g of a second precipitate is collected.
Part
What
Expre
тк
Su
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 9 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Sulfuric acid, H2SO4, reacts with sodium hydroxide, NaOH, in a neutralization reaction. What will appear on the product side of the correctly balanced chemical equation for this neutralization reaction?arrow_forwardIn the complete combustion of liquid octane, 2 C3H18 (1) + 25 O2 (g) --> 16 CO2 (g) + 18 H2O (g) determine the mass in grams of carbon dioxide produced if 12.2 grams of octane are used.arrow_forwardbalance and classification for ___ Sr(OH)2•8H2O (s) + ___ NH4Cl (s) → ___ SrCl2 (aq) + ____ NH3 (aq) + ____ H2O (l) _arrow_forward
- 3KOH+H3PO4⟶K3PO4+3H2O what mass of phosphoric acid in grams is needed to produce 2.15 mol of water?arrow_forward3 Cu + 8HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O In the above equation how many moles of water can be made when 138.6 grams of HNO3 are consumed?arrow_forwardHow many moles of Ba(OH)2 are present in 215 mL of 0.800 mol L-1 Ba(OH)2? Express your answer numerically in moles.arrow_forward
- An alkene having the molecular formula C6H₁2 is treated sequentially with ozone (03) and zinc/acetic acid to give the product/s shown. O Draw a structural formula for the alkene. H3C 1 • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. + ▾ CH3 will n [F ChemDoodleⓇ 16arrow_forwardWhat is the concentration (molarity) of a solution created by dissolving 83.7 grams of Be(NO3)2 to create a 1.0 solution?arrow_forwardWhat is the formula (solve for x) for the original hydrated sample of copper (II) sulphate? You weigh a sample of CuSO4 · XH2O. After you heat the sample, you find that the mass of the anhydrous salt is 1.47g. What is the value of x? CONTEXT Mass of just anhydrous compound = mass of anhydrous compound rod mass of beaker and 108.34g - 106.87g = 1.47g %3D %3D = original compound mass - anhydrous compound mass = 3.00g - 1.47g = 1.53g Mass of just waterarrow_forward
- 4. An old antacid commercial claimed that each tablet of their product could "neutralize 47 times its mass in stomach acid". The active ingredient in the antiacid tablet, NaAl(OH)₂CO3, reacts with HCl in stomach acid according to this balanced reaction: NaAl(OH)₂CO3 + 4 HCl →→ NaCl + AlCl³ + 3 H₂O + CO₂ How many moles of HCl can a 1.03 g of antiacid tablet neutralize if the tablet contains 0.246 g of the active ingredient? If stomach acid has a concentration of 0.14 M HCl, assuming the density of stomach acid to be similar to that of water (1.00 g/mL), what is the mass of stomach acid that the 1.03 g of antiacid tablet can neutralize? Does this number support the claim in the commercial?arrow_forwardComplete (where necessary) and balance the following equation. Write the stoichiometric equation, then write the equation in ionic and net ionic form. __ Cu(NO3)2 (aq) + __ NaOH (aq) → Cu(OH)2 (s) + __________arrow_forwardNa2CO3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY