Question
If the magnetic dipole moment of an atom is 1*10 ^24J/T, then this atom has a magnetic field of 10 T
how much does the potential energy change when placed?
Transcribed Image Text:mline Sınav
unika
el Fizik II - FIZ196
ru Listesi
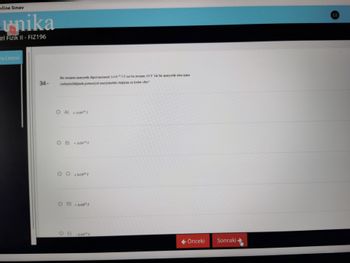
34-
Bir atomun manyetik dipol momenti 1x10 J/T ise bu atomun 10 T lik bir manyetik alan içine
yerleştirildiğinde potansiyel enerjisindeki değişim ne kadar olur?
OA) ± 1x10¹ J
O B)
+ Lx10 2¹ J
09
± [x10ª³ J
OD) + 1x10²5 J
OE)
Lx10¹3 J
← Önceki
Sonraki -
K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 6. A solid at temperature is placed in an external magnetic field B = 1 Tesla = 1 × 10¹ gauss. The solid contains weakly interacting paramagnetic atoms of spin 1/2, so that the energy of each atom is ±μB. a. If the magnetic moment is equal to one Bohr magneton, i.e., μ = h x 1.4 MHz/gauss, below what temperature must one cool the solid so that more than 75% of the atoms are polarized with their spins parallel to the external magnetic field? b. Suppose that one considered instead a solid which is free of paramagnetic atoms but contains many protons (e.g., paraffin). Each proton has spin 1/2 and a magnetic moment µp = h x 2.1 kHz/gauss. At the same temperature as you determined in part a., what fraction of protons will have their spins aligned parallel to the external magnetic field?arrow_forwardAssume the electron in a hydrogen atom is 53.0 pm from the nucleus of the atom, which consists of a single proton. (a) calculate the electrical force between the electron and the nucleus. (b) Calculate the gravitational force between the electron and the nucleus. (c) What is the ratio of the gravitational force to the electrical force?arrow_forwardThe question is below, much apprecatedarrow_forward
- What potential di fference is needed to accelerate a ⁹Be+³ ion from rest to a speed of 5 ×10⁵ m/s? NOTE: Feel free look up atomic number of Be on the periodic table. The mass number (given) is number of protons and neutrons combined.arrow_forwardIf an electron in an atom has an orbital angular momentum with m¡ = 2, what are the components (a) Lorb,z and (b) µorb,z? If the atom is in an external magnetic field that has magnitude 44 mT and is directed along the z axis, what are (c) the potential energy Uorb associated with the electron's orbital magnetic dipole moment and (d) the magnitude of the potential energy Uspin associated with the electron's spin magnetic dipole moment? If, instead, the electron has m₁ = -2, what are (e) Lorb,z, (f) Horb,z, (g) the potential energy Uorb associated with the electron's orbital magnetic dipole moment and (h) the magnitude of the potential energy Uspin associated with the electron's spin magnetic dipole moment? (a) Number i Units (b) Number Unitsarrow_forward5.6 1:01 IO 21%}0 4.ll Orange JO K/s A deuteron is accelerated from rest through a 10-kV potential difference and then moves perpendicularly to a uniform magnetic field with B = 1.6 T. What is the radius of the resulting circular path? (deuteron: m = 3.3 10-27 kg, q = 1.6 ´ 1019 C) a. 10 mm b. 19 mm с. 13 mm d. 9.0 mm e. 20 mm Previous page Next page - Sunday 20/12 Class Jump to... Quiz VIarrow_forward
- Consider a simple hydrogen atom where an electron orbits around a proton. Suppose a small magnetic field is applied perpendicularly to the plane of orbit. Show that if the orbit radius does not change, then the angular velocity of the electron would change by Δω = t eB 2marrow_forwardAs per Bohr model of a hydrogen atom for a stable orbit centripetal, Coulomb, and all forces should be in equilibrium. Therefore, for an electron with mass me and speed v₁ on the nth orbit with radius rn, (k being Coulomb/s constant) mevn = ke²/rn mevn² = ke²/rn mevn²/rn = ke²/rn 2.2 Ome²v² = ke²/r²arrow_forwardThe magnetism of permanent magnets arises because the inherent magnetic moment of electrons causes them to act like little compass needles. Protons also have an inherent magnetic moment, and this is the basis for magnetic resonance imaging (MRI) in medicine.Although a compass needle would prefer to align with a magnetic field, the needle can point in any direction. This isn’t the case for the magnetic moment of a proton. Quantum physics tells us that the proton’s energy must be quantized. There are only two possible energy levels—and thus two possible orientations—for protons in a magnetic field: E1 = -μB magnetic moment aligned with the field E2 = +μB magnetic moment aligned opposite the fieldwhere μ = 1.41 x 10-26 J/T is the known value of the proton’s magnetic moment. FIGURE 28.24 shows the two possible energy states. The magnetic moment, like a compass needle, “wants” to align with the field, so that is the lower-energy state.…arrow_forward
- F.j = (-e)(-v,k) × (B,i + B.k) ev Bzj. Solving for B gives F. Br 8.50x10 16 N = 1.13 T. Therefore B = 1.13 Ti – 0.772 Tk. The magnitude o A group of particles is traveling in a magnetic field of unknown magnitude and direction. You observe that a proton moving at 1.70 km/s in the +x-direction experiences a force of 2.10x10-16 N in the +y- direction, and an electron moving at 4.70 km/s in the eve (1.60x10 19 C)(4700 m/s) is B = VB? + B = (1.13 T)² + (-0.772 T)² = 1.37 T. -z-direction experiences a force of 8.50x10-16 N in the +y-direction. Part B For related problem-solving tips and strategies, you may want to view a Video Tutor Solution of Magnetic force on a proton. What is the direction of the magnetic field? (in the xz-plane) Express your answer in degrees. 0 = 60.59 ° from the -z-direction to the +x-direction Submit Previous Answers Request Answer X Incorrect; Try Again; 9 attempts remainingarrow_forwardIf the speed of the electron in Example 19-4 were 7.3 * 105m>s,what would be the corresponding orbital radius?arrow_forwardChapter 29: Magnetic Fields We studied how a moving charge would react in a magnetic field, which extends naturally to current where charges drift (hence moves) in a magnetic field. One special case of this is the torque due to current loops in magnetic fields: 7= μx B= IAX B since we defined the magnetic moment of a current loop as μ = IĀ. estimate the current that an electric car would have to provide to its motor such that it can do 0-100 kph in 3 seconds. Some useful hints for guidance are below: (1) . Find the acceleration using kinematics With the acceleration, what would be the torque needed for the motor to apply to the wheel? You need to estimate yourself the size of the wheel, as well as the size of the motor hub that is connected to the wheel. A simple electric motor has a permanent magnet, say of strength of 1 Tesla, and a coil acting as many current loops. You need to estimate the number of loops, you can google it just come up with a reasonable number. ● From this and…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios