
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Question 4 of 7 >
O Macmillan Learning
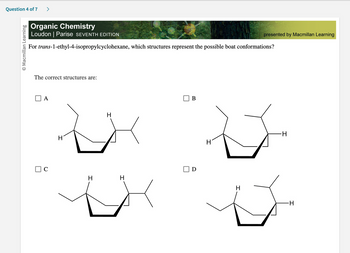
Organic Chemistry
Loudon | Parise SEVENTH EDITION
For trans-1-ethyl-4-isopropylcyclohexane, which structures represent the possible boat conformations?
The correct structures are:
A
C
H
H
H
H
B
D
presented by Macmillan Learning
H
-H
H
D
I
-H
Transcribed Image Text:Question 3 of 7
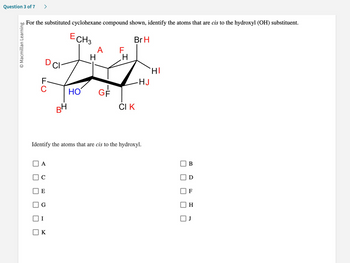
O Macmillan Learning
For the substituted cyclohexane compound shown, identify the atoms that are cis to the hydroxyl (OH) substituent.
D CI
FC
A
E
G
BH
K
ECH3
ات
HO
A F
-LL
GF
H
Identify the atoms that are cis to the hydroxyl.
Br H
CI K
HI
HJ
B
F
H
J
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of these is the most stable conformation of butane, looking down the C2-C3 bond? HCCH3 HCH3 CH3 CH3 CH3 HH H. CH3 A В C O B O D O Aarrow_forward4 CI H3CH₂C Consider the following structure. Convert the Newman projection to a bond-line formula with wedges and dashes showing the stereochemistry. CH3 H3 CH ₂ C CI is cs protected and ma H F Polyclooctane R Please check if the diagram C is correct CH3 fap!!!! H K Harrow_forwardE ||| CH3 H H H CH3 H H₂ H H H CH3 CH3 Question 8 of 34 Identify the Newman projection that depicts the gauche conformation of butane. H 11 H H. HCH₂ H V H₂C CH₂ IV H CH ₂ H H CH₂ CH H H CH3 A) I B) II C) III D) IV E) V Submiarrow_forward
- 4. Draw the Newman projection of the conformer below as it is drawn looking down the indicated C-C bond. Then draw the most stable conformer and the least stable conformer as Newman projections. PH. CH3 H H CI Newman of Drawn Newman of Most Newman of Least Conformer Stable Conformer Stable Conformerarrow_forwardPlease don't provide handwriting solutionarrow_forwardGiven cyclohexane in a chair conformation, draw the most stable isomer and conformation of 1,3-dibromocyclohexane by adding in the missing atoms. H H txt H H- H Answer Bank Brarrow_forward
- Q8 Conformational Analysis of Cycloalkanes In this question you will look at the conformations of the following molecule: CH3 C В F CH3 D -E A H3C F I II Energy Costs for Interactions in Alkane Conformers: Computed Energy Cost (kJ/mol)* 3.8 Interaction H+H eclipsed H+CH3 eclipsed CH3+CH3 eclipsed CH3+CH3 gauche H»CH(CH3)2 gauche H CH(CH:)2 eclipsed CH3+CH(CH3)2 gauche CH3+CH(CH3)2 eclipsed HeC(CH:)3 eclipsed CH3+C(CH3)3 gauche CH3+C(CH:)3 eclipsed H+I eclipsed I+CH3 gauche I+CH3 eclipsed "Values computed with Gaussian09 using B3LYP/6-31G(d) for structure optimization and B3LYP/6-311+G(2df,3p) for the conformation energy. The calculations were performed in the gas phase (no solvent). *This result for t-butyl is a bit peculiar given that the value is less than that for isopropyl, but there is an explanation that I can tell you about in office hours! 4.8 12.6 3.7 (0.0) 3.1 3.6 17.3 2.8 13.6 18.7 6.7 1.2 15.5 Steric Strain in Monosubstituted Cyclohexanes: 1,3-Diaxial strain Y (kJ/mol)…arrow_forwardWhich one of the structures below is the most stable / lowest energy confor of the cis disubstituted-cyclohexane? %3D II IV OII O IVarrow_forwardNeed help on this asaparrow_forward
- Please don't provide handwriting solutionarrow_forward3-44 Draw the two chair conformations of each compound and label the substituents as axial and equatorial. In each case, deter- mine which conformation is more stable. (a) cis-1-ethyl-2-isopropylcyclohexane (c) cis-1-ethyl-3-methylcyclohexane (e) cis-1-ethyl-4-methylcyclohexane (d) (b) trans-1-ethyl-2-isopropylcyclohexane trans-1-ethyl-3-methylcyclohexane trans-1-ethyl-4-methylcyclohexane (f)arrow_forwardOrganic Chem HWarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY