Question
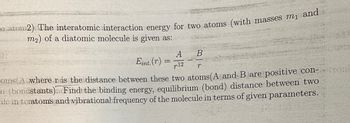
Transcribed Image Text:o atom2) The interatomic interaction energy for two atoms (with masses m₁ and
m2) of a diatomic molecule is given as:
A
B
Eint. (r)
12
r
oms(A where r is the distance between these two atoms (A and B are positive con-troni
(bondstants) Find the binding energy, equilibrium (bond) distance between two
ilc in teratoms and vibrational frequency of the molecule in terms of given parameters.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Similar questions
- p9C.1 Familiarity with the magnitudes of overlap integrals is useful when con- sidering bonding abilities of atoms, and hydrogenic orbitals give an indication of their values. (a) The overlap integral between two hydrogenic 2s orbitals is 1 ( ZR ZR +. 2а, " 12 а, 1 + ZR 240 a, -ZR/Z0 S(2s, 2s)={1+ Plot this expression. (b) For what internuclear distance is S(2s,2s) = 0.50? (c) The side-by-side overlap of two 2p orbitals of atoms of atomic number Z is ZR 1 ( ZR ZR S(2p,2p) ={1+ 10 a, 2a, 120 a. Plot this expression. (d) Evaluate S(2s,2p) at the internuclear distance you calculated in part (b).arrow_forwardThe net potential energy E_N between two adjacent ions is sometimes represented by the expression: E_N = -C/r + D times exp (-r/rho) in which r is the interionic separation and C, d, and rho are constants whose values depend on the specific material. Derive an expression for the bonding energy E_0 in terms of the equilibrium interionic separation r_0 and the constants D and rho. Derive another expression for E_0 in terms of r_0, C and rho.arrow_forwardSAMPLE PROBLEMS & SOLUTIONS Example 1: The interacting potential energy between two ions is represented by the following expression: -1.252 5.625 x 10-6 U = ..Eqn(1) r8 Where (r) is the distance in nm (1nm = 10-m) (U) is in kJ Calculate: a) The equilibrium distance ro in nm. b) The minimum bond energy in kJ. c) The maximum interionic force for the bond in kN.arrow_forward
- The number of normal modes of vibration depends on the shape of the molecule, but not on the number of atoms in the molecule. True Falsearrow_forward6. Consider the bond vibration of a homo-atomic diatomic molecule. In the harmonic approximation the vibrational energy levels are given by, E₂ = (v + 1 ) Aes -1 S Where v = 0,1,..., ∞ and w = 6.1 × 10¹4 s−¹. Let us assume this vibrational mode is IR active. A photon of energy E = hc/ is absorbed by the molecule and induces a fundamental vibrational transition. (a) What is the wavelength of the resulting IR absorption peak in nm?arrow_forwardQ2/ For the harmonic oscillator in classical limit, if the partition function is given by Z(B) = 1/(ßħw)N, find the entropy and internal energy. Q3/ Prove that F = -KTlnz .arrow_forward
arrow_back_ios
arrow_forward_ios