
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:* Dementia Frien x
(2 unread) - dtɛ x
Lobby | Top Hat
Watch Gilmore
A www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvdWKW_BBZZ16tTytly
Spotify Web Playe... M Common Ethical D..
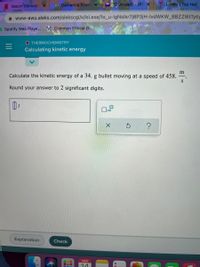
O THERMOCHEMISTRY
Calculating kinetic energy
m
Calculate the kinetic energy of a 34. g bullet moving at a speed of 458.
Round your answer to 2 significant digits.
Explanation
Check
MAR
14
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the amount of heat needed to boil 68.6 g of water (H₂O), beginning from a temperature of 37.2 °C. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol. 0 Xarrow_forward110.0 g of iron is heated to 92.7 degrees C and then place in a container of water at 22.3 degrees C. The final temperature of the water and iron is 26.3 C. What is the mass of water in the container, assuming that all the heat lost by the iron is gained by the water. (significant figures needed) thank you!arrow_forwardCalculate the amount of heat needed to boil 34.0 g of water (H₂O), beginning from a temperature of 51.4 °C. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol. x10 X 1/5 Ś ? 000 Ararrow_forward
- Calculate the kinetic energy of a 0.15kg baseball moving at a speed of 45.0ms. Round your answer to 2 significant digits.arrow_forward(CONTINUED ON NEXT PAGE) 4. Let's say that the full volume of your flask is 325 ml. You heat the flask of air in a hot water bath at 89.0°C according to your thermometer. You then inverti into the cold water bath at 5.0°C Water enters the flask as the volume of the gas shrinks. a) Theoretically, what should the volume of the gas be after it sits in the cold water bath? b) You find that during your experiment that 65.0 mL of water has entered the flask. What is the experimental volume of the gas? c) What is your percent error for this measurement?arrow_forwardA sheet of gold weighing 9.0 g and at a temperature of 13.8 °C is placed flat on a sheet of iron weighing 20.2 g and at a temperature of 57.8 °C. What is the final temperature of the combined metals? Assume that no heat is lost to the surroundings. Be sure your answer has the correct number of significant digits. GC ☐ x10 ☑ ملام 18 Ar 日。 ☑arrow_forward
- A975.7J of heat was applied to raise the temperature of a beaker of water from 47.0 oC to 63.0 oC. Assuming all the heat went into heating the water only, calculate the mass of the water in the beaker. (The specific heatof water is 1cal/goc or 4.184J/goc) Give the answer in the correct significant figures.arrow_forwardA 1,581,819 g car is traveling 0.823 m/s. Calculate the kinetic energy of the car in J. Do not include units in your answer. Give the answer as a whole number.arrow_forwardWhat amount of heat, in J, is required to raise the temperature of 391.2 g of lead by 14.3°C? The specific heat of lead is 0.128 J/g°C. Give your answer in standard notation to 0 decimal places.arrow_forward
- Convert 36 kcal to J Record the answer in scientific notation (e.g. 2.10E3J)arrow_forwardUse the de Broglie relationship to determine the wavelengths of the following objects.arrow_forwardA 355 g sample of water goes 85 degrees C to 42 degrees C. Calculate the amount of heat energy (in calories) involved in this process. Report the answer in significant figures.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY