
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
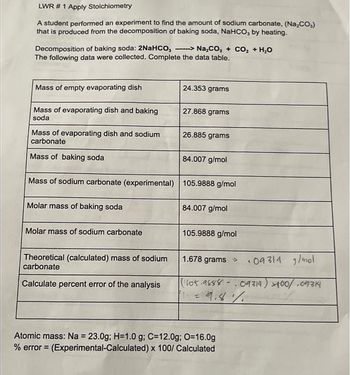
Transcribed Image Text:LWR # 1 Apply Stoichiometry
A student performed an experiment to find the amount of sodium carbonate, (Na₂CO₂)
that is produced from the decomposition of baking soda, NaHCO, by heating.
Decomposition of baking soda: 2NaHCO,> Na₂CO, + CO₂ + H₂O
The following data were collected. Complete the data table.
Mass of empty evaporating dish
24.353 grams
Mass of evaporating dish and baking
27.868 grams
soda
26.885 grams
Mass of evaporating dish and sodium
carbonate
Mass of baking soda
84.007 g/mol
Mass of sodium carbonate (experimental) 105.9888 g/mol
Molar mass of baking soda
84.007 g/mol
Molar mass of sodium carbonate
105.9888 g/mol
1.678 grams >
Theoretical (calculated) mass of sodium
carbonate
09314 g/mol
Calculate percent error of the analysis
(1105.9888-09314) +100/.09314
= 9.8%
1 =
Atomic mass: Na = 23.0g; H=1.0 g; C=12.0g; O=16.0g
% error = (Experimental-Calculated) x 100/ Calculated
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- [References] nelling salts," which are used to revive someone who has fainted, typically contain ammonium carbonate, (NH4)2 CO3 . Ammon. ponate decomposes readily to form ammonia, carbon dioxide, and water. The strong odor of the ammonia usually restores conscio person who has fainted. The unbalanced equation is (NH4)2 CO3(s) → NH3(9) + CO2(g) + H2O(g) alculate the mass of ammonia gas that is produced if 2.40 g of ammonium carbonate decomposes completely. g NH3 Submit Answer Try Another Version 1 item attempt remainingarrow_forwardWhat is the easiest process to get to the result?arrow_forward62. Antacid Fizz When an antacid tablet dissolves in water, the fizz is due to a reaction between sodium hydrogen carbonate (NaHCO3), also called sodium bicarbonate, and citric acid (H₂CH₂O₂). 3NaHCO3(aq) + H₂CH₂O,(aq) → 3CO₂(g) + 3H₂O(l) + Na, C₂H₂O₂(aq) How many moles of Na₂C,H,O, can be produced if one tablet containing 0.0119 mol of NaHCO is dissolved?arrow_forward
- Consider the reaction of copper(II) oxide with aluminum metal to produce copper metal and aluminum oxide: 3 CuO(s) + 2 AlI(s) → 3 Cu(s) + Al2O3(s) If 7.99x1023 atoms Al react with an excess of CuO, what mass of Cu(s) is produced? Assume the reaction goes to 100% completion. Enter your response in decimal notation to three significant figures. Answer: Enter Value g.arrow_forwardTo produce zinc metal, carbon monoxide reacts with zinc (I1) oxide according to: Zno + CO - Zn + CO2 The carbon monoxide, however, is initially prepared from: 2C+ 02 → 200 How many grams of zinc metal (Zn) may be prepared if If 81.0g of C is used with excess amount of ZnO? Your answer should be numerical and neglect units (e.g., if you calculate 3.5g then input 3.5).arrow_forwardThe reaction below takes place in water at room tempeture. Balance the reaction and write the correct state of matter for the substance. CaCI_2(aq) + (NH_4)_3PO_4 -> Ca_3(PO_4)_2 + NH_4CIarrow_forward
- Soda ash (sodium carbonate) is widely used in the manufacture of glass. Prior to the environmental movement much of it was produced by the following reaction. CaCO3 + 2 NaCl → Na2CO3 + CaCl2 Unfortunately, the byproduct calcium chloride is of little use and was dumped into rivers, creating a pollution problem. As a result of the environmental movement, all of these plants closed. Assume that 125g of calcium carbonate (100.09 g/mol) and 125 g of sodium chloride (58.44 g/mol) are allowed to react. Determine how many grams of useful sodium carbonate (105.99 g/mol) will be produced. How many grams of useless calcium chloride (110.98 g/mol) will be produced? You should also determine how many grams of excess reactants are left (indicate which one is the limiting reactant)arrow_forwardCalculate the mass(in grams) of the product formed when 15.40g of Cl_2(g) completely reacts. Assume that there is more than enough of the other reactant. 2 K(s) + Cl _2(g) --> 2KCl(s)arrow_forwardThe extraction of aluminum metal from the aluminum hydroxide found in bauxite by the Hall-Héroult process is one of the most remarkable success stories of 19th century chemistry, turning aluminum from a rare and precious metal into the cheap commodity it is today. In the first step, aluminum hydroxide reacts to form alumina Al2O3 and water: 2Al(OH)3(s) + Al2O3(s) -> 3H2O(g) In the second step, alumina Al2O3 and carbon react to form aluminum and carbon dioxide: 2Al2O3(s) + 3C(s) + 4Al(s) -> 3CO2(g) Suppose the yield of the first step is 74.% and the yield of the second step is 76.% . Calculate the mass of aluminum hydroxide required to make 4.0kg of aluminum. Be sure your answer has a unit symbol, if needed, and is rounded to the correct number of significant digits.arrow_forward
- Name Lab Partner Section # Date Pre-Lab Assignment: Stoichiometry Lab 1. In a lab experiment similar to the one you are going to do, a student slowly added hy- drochloric acid (HCI) to an evaporating dish containing magnesium carbonate MgCO3. a. Complete and balance the following equation for the reaction between magnesium carbonate and hydrochloric acid. MgCO3 (s) + HCl (aq) → (aq) + H₂CO3(aq) (When writing the formula for the missing product did you consider the charges of the ions?) b. When carbonic acid is formed in a chemical reaction, it is unstable and immedi- ately decomposes into carbon dioxide and water. Rewrite the equation above to show the final products using this information.arrow_forwardRemaining Time! 02:29:4. Consider the balanced chemical equations shown below. When a 6.00 g sample of a mixture of iron (Fe) and aluminum (Al) is treated with excess HCl(aq), 0.177 moles of H2 are obtained. What is the mass percentage of Fe in the original mixture? Choose the closest answer. Fe(s) + 2 HCI(aq) FeCl2(aq) - + H2(g) 2 Al(s) +6 HC((aq) 2 AICI3(aq) + 3 H2(g) 96 % 69 % 47 % 33 % 17 % Rack Question Menu - O Oarrow_forward+ BIUA Normal text Arial 12 二 三1|-山- 1 1 3. 5. If 10.0 gram of aluminum chloride are decomposed, how many molecules of Cl, are produced? | AICI, → Al + Cl2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY