
A container holds a sample of ideal gas in thermal equilibrium, as shown in the figure. (Figure 1) One end of the container is sealed with a piston whose head is perfectly free to move, unless it is locked in place. The walls of the container readily allow the transfer of energy via heat, unless the piston is insulated from its surroundings.
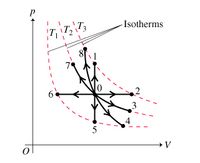
Refer to the pV diagram presented to answer the questions below. (Figure 2) In each case, the piston head is initially unlocked and the gas is in equilibrium at the pressure and volume indicated by point 0 on the diagram.
Starting from equilibrium at point 0, what point on the pV diagram will describe the ideal gas after the following process?
"Lock the piston head in place, and hold the container above a very hot flame."
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

- Use the data from this table of thermodynamic properties to calculate the values of AS for each of the reactions at 25 °C. C(s, graphite) + H, O(g) CO(g)+ H,(g) AS = N, (g) + 3 H, (g) - 2 NH,(g) AS = K Question Source. McQuararrow_forwardA 0.1 kg block of pure copper at a temperature of 250°C is dropped into a 4°C water bath. The bath contains 0.03 m^3 of water and is well insulated. Find the final temperature after the block and the water reach thermal equilibrium. (It is reasonable to assume the following: the specific heat of water is equal to the value at 4°C; the specific heat of the material in the block can be taken to be a constant value evaluated at a temperature mid-way between the initial temperatures of the block and the water.)arrow_forwardCan someone please answer part a and part b. but please transform the PV diagram to a TS diagram first and express Ti in terms of Cp, Cv, TL, and THarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





