
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Pleasedon't provide handwritten solution...

Transcribed Image Text:choose the correct intermediate and continue the mechanism showing all
intermediatesand electron motions needed to produce the product of that reaction
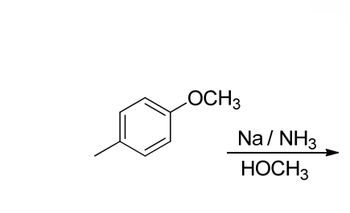
Transcribed Image Text:LOCH3
Na/NH3
HOCH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- show-all-working-explaining-detailly-each-step Answer should be typewritten using a computer keyboard.arrow_forwardPb(NO3)2 is placed in water. Please predict the physical state using our solubility rules from class. Select one: a. aqueous - (aq) b. liquid - (l) c. gas - (g) d. solid - (s) e. plasma - (p)arrow_forwardnok Experiment 1: Laboratory Report Tixed Salt Solutions 4. For ench renction! A> urite a word equntion b) vrite the balanced moleculas eauntion c) write the total ced J) vritc the balmced net ionic enuntron ionsc enuntron 1, Renctants.' Ag Noz caz) NazCOz cra) 2. Renctmts i Tese co CNOZ)2 Na z S cn> 3. Renctats ; cuCNOg)2arrow_forward
- Please ansuer the followiveg themocheawishry questian. Starchiometny or (and Vimitinal excess reagont calculations midht be mecessany. Please shew all wark and explain essay. Steps in details a) Haw much energu is produced when 93.5 Oxygan reacts with 13.2q hydrapen in the followng reaction? of grams 2H2 + Oz Đ2H2O Att= -572 KJ b) How many grans of magmesiaom sulfate woud be praduced fram the fallowine reaction if 176g of energy is absorbed the reactian: by Alz (sou)o f 3Mq Iz+2AI G+3Mg(304) AM= +722 KJarrow_forwardBalance each of the following equations. (State indicators are not required) Please answer (a) through (o). (a) Cdf2 + NaBr --> CdBr2 + NaF (b) Cr + F2 --> CrF3 (c) Ca + H2O --> Ca(OH)2 + H2 (d) Bi(NO3)3 + Na2S --> Bi2S3 + NaNO3 (e) C2H5OH + O2 --> CO2 + H2O (f) V + S8 --> V2S5 (g) LiNO3 + Li --> Li2O + N2 (h) Ca3(PO4)2 + H2SO --> CaSO4 + H3PO4 (i) PH3 + O2 --> P2O5 + H2O (j) Ba + Ag3PO4 --> Ba3(PO4)2 + Ag (k) Ca(Clo3)2 --> CaCl2 + O2 (l) C12H22O11 + O2 --> CO2 + H2O (m) Ca2C + H2O --> Ca(OH)2 +CH4 (n) NH4Br + BaO --> NH3 + BaBr2 + H2O (o) LiAlH4 + BF3 --> LiF + AlF3 + B2H6arrow_forwardPlease don't provide handwritten solutionarrow_forward
- barkamishen seslb to doing Bittetsbau nA sideT oibore peivab ot etsimodo it as lendas suchombre or to agninnigod adr oldmseen polls anoitoleaimora ni baya Solovi immediabudinng enoitonen Isolmarlo lo coqyt Istoresbout wot ybuta lliw s Croborecho ofnino2s1 ayow ribi of 5) Analysis of 1.618 g of a hydrate salt gave the following information. Iliw Pb: 0.884 g, C: 0.205 g, H: 0.026 g, O: 0.273 g, and water: 0.230 g.o Inters What is the formula of the hydrate. sob a ogrobimu snocheoid bas in botser asce ed with asmolo s nad oson inmoasigaib to savi Inomalo srit 'il bogoo oinai is gaininos nouutos of babbe smogod bis noi si di autosis syneriozs Irw ticodulos ni noi ni nobnice to bilo 25ml produced flash and poppe carolionoriconic lieth noitonen inomossigen sigme yd elas Jabot ovi HA obrtw scale 6 10 enolonias pozemol adi ni ailuas, noitufos at devan gods got abou odregos bazum o obnoldo miuiboa bar otain rovlie to anoituloa asrl W SiT moironen insmoelor siduob a lo loss of an emot…arrow_forward0 Cals) + NAC lag) – O White the balanced molecular chemical equation for the reachion f Solid lithium with aqueo us Chromium (111) acetat: 4 no iea chun occurs, write UR. Idaite a balanced molecular Chemical equahion for the Solia Sodium with aqueous Zinc perchlorite . 7 no geaction ccuns,arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY