
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
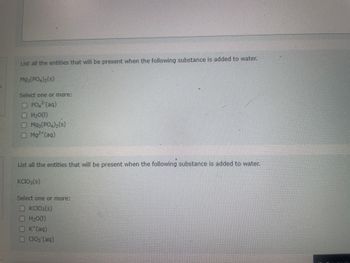
Transcribed Image Text:List all the entities that will be present when the following substance is added to water.
Mg3(PO4)2 (S)
Select one or more:
PO² (aq)
H₂O(1)
□
Mg3(PO4)₂ (s)
Mg²+ (aq)
List all the entities that will be present when the following substance is added to water.
KCIO3(s)
Select one or more:
KCIO3(s)
H₂O(1)
K*(aq)
ClO₂ (aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You need 500 mL of a 0.5 M iron(III) chloride solution. You only have a 3.0 M FeCl3 solution on hand. How much of the higher concentrated stock solution do you need to make the desired solution?arrow_forward4. Which of the following aqueous soluti ons is the best conductor of electricity? A 0.10M CH,OH R LOM CH,OH C. 0.10 M NaOH D. 1.0M NaDHarrow_forwardThe maximum contaminant level of cyanide (CN) in drinking water as set by the the Environmental Protection Agency (EPA) is 0.00020 g · L. Express this concentration in parts per million (ppm). Assume the density of water is 1.00 g/mL. concentration: Ppmarrow_forward
- "Rubbing alcohol" is a mixture that is 70.% isopropyl alcohol (C3H8O) in water. The mixture has a density of 0.79 g/mL at 20°C. What is the concentration of the isopropyl alcohol in molarity? The molar mass of isopropyl alcohol is 60.11 g/mol. a. 11.6 M b. 13.1 M c. 9.2 M d. 30.7 Marrow_forwardWrite down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H2O).arrow_forwardQUESTION 10 (4b-401-1.53-1) An intern working in a research lab knocks over a beaker containing an important solution. The beaker originally contained 1.50 L of a 1.53 M solution, but after it spilled it only contained 1 L of solution. The intern decides to hide his mistake by refilling the beaker to contain 1.50 L again by adding pure water (not realizing that this will dilute the solution). What is the new concentration of the solution? Show all work and give your answer with three sig figs. Click Save and Submit to save and submit. Click Save All Answers to save all answers. Save All Answer 1600 4,104 PAGES APR O P W MacBook Air DII DD 80 000 000 F7 F8 F9 F10 F2 F3 F4 F5 F6 F1 #3 $ & 2 3 4 6 7 8. W E T Y P R ... 2.arrow_forward
- a) Write the balanced equation for the reactionarrow_forward[References] A sample of an industrial waste water is analyzed and found to contain 48.5 ppb Co³+. How many grams of colbalt could be removed from 1.98×10³ kg of this waste water? grams Coarrow_forwardConsider the following balanced chemical equation: Mg(OH)2 + 2HCl → 2H2O + MgCl2 How many grams of HCl would be required to neutralize 50.0 grams of Mg(OH)2?arrow_forward
- What is the molarity of a solution in which 4.00 mol of potassium chloride (molar mass 74.55 g/mol) is dissolved in 3.00 L of solution? O 2.67 M 4.00 M 6.00 M O 1.33 M MacBook Air D00 20 F3 F10 esc F4 F5 F7 F8 F9 F6 F1 F2 @ #3 2$ % 2 3 6 8 Q W E R Y ーのarrow_forwardHow many moles of CsBr are contained in 244 mL of 0.135 m CsBr solution? The density of the solution is 1.22 g/mL. Choose the correct answer from below. Thank you -3.91 × 10-2 mol CsBr -2.21 × 10-2 mol CsBr -4.31 × 10-2 mol CsBr -2.32 × 10-2 mol CsBr -3.29 × 10-2 mol CsBrarrow_forwardHow many milliliters of a 0.2228 M H3PO4 solution are required to neutralize 3.14 g of KOH? Molar Mass: H3PO4 = 97.99 g/mol KOH = 56.11 g/mol H3PO4 + 3 KOH → 3 H2O + K3PO4arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY