
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
A chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of chloroform, diethylamine, ethanolamine, acetone, and pentane.
The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from his collection of Material Safety Data Sheets (MSDS), the chemist finds the following information:
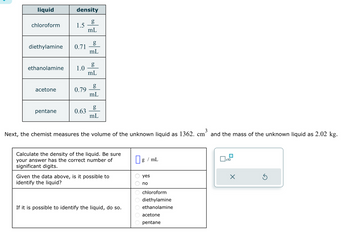
Transcribed Image Text:liquid
chloroform
density
diethylamine 0.71
acetone
1.5
ethanolamine 1.0
pentane
0.79
0.63
mL
g
mL
mL
g
mL
g
mL
Next, the chemist measures the volume of the unknown liquid as 1362. cm³ and the mass of the unknown liquid as 2.02 kg.
3
Calculate the density of the liquid. Be sure
your answer has the correct number of
significant digits.
Given the data above, is it possible to
identify the liquid?
If it is possible to identify the liquid, do so.
g/mL
yes
no
chloroform
diethylamine
ethanolamine
acetone
pentane
x10
X
Ś
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of methyl acetate, chloroform, ethanolamine, diethylamine, and tetrahydrofuran. The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from his collection of Material Safety Data Sheets (MSDS), the chemist finds the following information: liquid methyl acetate chloroform ethanolamine diethylamine tetrahydrofuran density 0.93 g ml 1.5 g ml 1 -1 1.0 g ml 1 0.71 g ml 0.89 g ml. Next, the chemist measures the volume of the unknown liquid as 1005. cm' and the mass of the unknown liquid as 1.49 kg. Calculate the density of the liquid. Be sure your answer has the correct number of significant digits. Given the data above, is it possible to Identify the liquid? If it is possible to identify the liquid, do so. 0 gml. yes no methyl acetate chloroform ethanolamine…arrow_forwardSome people like to make their own hand lotion because it can be less expensive than buying it and they have the ability to add only the fragrances that they like. A recipe for hand lotion calls for mixing 253 g of water (H₂O) with 264 g of glycerin (C3H8O3). This recipe makes a total of 685 mL of hand lotion. Incorrect. What is the mass percent of glycerin in the hand lotion? i ! % Hint The mass percent is the concentration of the solution as the percent of solute in a given mass of solution. Assistance Usedarrow_forwardBefore investigating the scene, the technician must dilute the luminol solution to a concentration of 5.00 x 10-2 M. The diluted solution is then placed in a spray bottle for application on the desired surfaces. How many moles of luminol are present in 2.00 L of the diluted spray? Express your answer with the appropriate units.arrow_forward
- A chemist prepares a solution of potassium iodide (KI) by measuring out 175. μmol of potassium iodide into a 200. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mmol/L of the chemist's potassium iodide solution. Round your answer to 3 significant digits. mmol L 0 x10 X 3arrow_forwardA chardonnay wine is 13.5 percent by mass alcohol. If we consume 24 fluid oz of wine, and the density of the wine is 0.982 mg/mL, how much alcohol was consumed? 09.6 x 103 g alcohol O 301 g alcohol O 94 g alcohol O 106 g alcohol O 13.3 g alcoholarrow_forwardA chemist prepares a solution of nickel (II) chloride (NiC1₂) by measuring out 250. g of nickel (II) chloride into a 400. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's nickel (II) chloride solution. Be sure your answer has the correct number of significant digits. mol/L x10 Śarrow_forward
- A chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of dimethyl sulfoxide, glycerol, chloroform, acetone, and ethanolamine. The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from her collection of Material Safety Data Sheets (MSDS), the chemist finds the following information: liquid density dimethyl sulfoxide 1.1 olo 3 cm Ar glycerol 1.3 3 cm chloroform 1.5 3 cm acetone 0.79 3 cm ethanolamine 1.0 3arrow_forwardA chemist prepares a solution of aluminum chloride AlCl3 by measuring out 32.μmol of aluminum chloride into a 150.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in μmol/L of the chemist's aluminum chloride solution. Be sure your answer has the correct number of significant digits.arrow_forward) A 1 gallon bottle of a certain brand of bleach costs $1.49. Determine the cost to buy enough bleach to supply 100.0 grams of active ingredient (NaOCl), assuming 6.25% active ingredient by mass and a density of 1.05 g/ml.arrow_forward
- A chemist prepares a solution of silver perchlorate (AgClO,) by weighing out 2.40 kg of silver perchlorate into a 500. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in g/dL of the chemist's silver perchlorate solution. Be sure your answer has the correct number of significant digits. x10 đLarrow_forwardDimensional Analysis is a way of doing numerical "book-keeping" when converting quantities or performing calculations. • When converting quantities from one unit to another, conversion factors are used. Solving with Dimensional Analysis and Multiple Units: If I am in Canada where the price of gas is $1.022 USD·L1, how much will it cost me to fill up my gas tank if I travelled 125 km? • Let's also assume that my car gets an average of 30.0 miles/gallon.arrow_forwardA chemist prepares a solution of nickel(II) chloride NiCl2 by measuring out 97.μmol of nickel(II) chloride into a 150.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mmol/L of the chemist's nickel(II) chloride solution. Round your answer to 2 significant digits.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY