
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
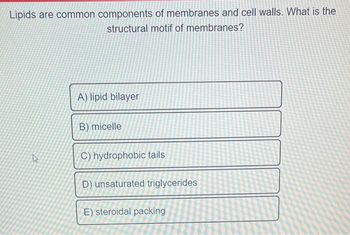
Transcribed Image Text:Lipids are common components of membranes and cell walls. What is the
structural motif of membranes?
13
A) lipid bilayer
B) micelle
C) hydrophobic tails
D) unsaturated triglycerides
E) steroidal packing
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- When phospholipids are carefully mixed with water they are observed to exhibit which of the following properties? a) Spontaneous organization into stable structures within the water environment, b) Orientation of the Phosphate parts of the molecule away from fatty acid (non-polar) environment of a bilayer, c) Hydrophilic orientation of the ionic section of the phospholipid molecules to the polar portions of the water molecules, d) All are true, e) None are true.arrow_forwardWhat components are important for making a lipid membrane? please explain the answer and choice the right one a)C. acyl carrier proteins b)B. signal sequences c)A and C d)A and B e)A. flippasesarrow_forwardA transmembrane protein contains a stretch of amino acids that crosses the plasma membrane. The side chains of the amino acids that associate with the phospholipid tails are: A) electronegative B) hydrophobic C) ionic D) polar E) hydrophilicarrow_forward
- The movement of water along a concentration gradient through a semi-permeable membrane: A) osmosis B) endocytosis C) diffusion D) active transport Question 4 Which bonds are created when the primary structure of a protein forms? OA) peptide bonds B) ionic bonds C) glycosidic bonds D) hydrogen bondsarrow_forwardWhen a living cell, which has an outer membrane made of phospholipids, is placed in an aqueous (water) solution containing K+ and Na+ ions, one observes that these ions appear inside the cell. One would hypothesize that a) the phospho groups moved the ions through the Fatty Acids. b) the cell would swim away. c) H2O dissolved the membrane d) lipids had moved in and out of the cell through the water e) there must be a special mechanism moving the ions because of the properties of Fatty Acids.arrow_forwardIn an adipose cell, after being synthesized by the ribosomes and the rough endoplasmic reticulum a protein involved in hydrolysis of lipid could be transported to the: a) mitochondrion, b) smooth endoplasmic reticulum, c) Golgi apparatus, d) Chloroplasts, e) nucleusarrow_forward
- Which of the following is an example of secondary protein structure?A) DipeptideB) TriglycerideC)α -helixD) Amino acidE) Fatty acidarrow_forwardWater-soluble proteins such as myoglobin tend to fold such that: A) hydrophobic amino acids R-groups are on the interior of the protein and hydrophilic groups are on the outside OB) all peptides form hydrogen bonds with water. C) hydrophilic amino acid R-groups are on the interior of the protein and hydrophobic groups are on the outside. O D) hydrophilic and hydrophobic amino acid R-groups form hydrogen bonds with each other.arrow_forwardA) Briefly describe the basic structure of phospholipids and explain how they can provide an effective barrier against the unregulated movement of molecules into or out of cells. B) Not all phospholipids are identical to one another. Describe two components (parts) of that can be altered to create variation between different phospholipids.arrow_forward
- What type of bonds/forces stabilize protein secondary structure? A) ionic (electrostatic) B) hydrogen bonds C) hydrophobic forces D) A and C O E) A, B and Carrow_forwardWhich is an appropriate statement of involvement of the hydrophobic effect in protein folding? A) Nonpolar portions interact with polar portions in the interior of the protein. O B) Nonpolar portions of the molecule associate with one another in the interior of the protein. OC) Nonpolar portions of the molecule can be placed on the surface of the molecule only if hydrogen bonded to water. OD) Polar portions of the molecule are generally exposed to solvent to interact effectively with water.arrow_forwardYou have a cellulose sack that is impermeable (i.e. NOT permeable) to starch molecules and is filled with a solution, comprised of 20% starch. It is placed in a container of 100% distilled water. a) What term describes the solution in the sack compared to the solution surrounding it? i) HYPERTONIC ii) HYPOTONIC iii) ISOTONIC b) In which direction will the water molecules move faster? i) INTO THE CELL ii) OUT OF THE CELL iii) SAME IN BOTH iv) NOT AT ALL c) In which direction will the starch molecules move? i) INTO THE CELL ii) OUT OF THE CELL iii) SAME IN BOTH iv) NOT AT ALLarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON