
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Name and draw structural formulas for all possible monohalogenation products that might be formed in the following reaction .
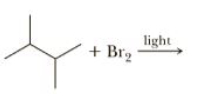
Transcribed Image Text:light
+ Br2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What change can be made to allow for a faster rate of reaction if poor solubility of KOH in ethanol slowed the rate and led to slow deprotonation of dibenzyl ketone?arrow_forwardhow many structurally isomeric products form in the free radical bromination of hexane?arrow_forwardwrite the product of secondary alkyl bromide reacting with ethoxide ionarrow_forward
- 1. What changes in color occur when bromine reacts with an alkene?arrow_forwardWhat is the overall % yield of triphenylmethane from benzophenone given the percentage yield of each step of the reaction sequence as follows? Give only two significant digits. If the answer is a whole number with two digits, do not include the decimal. 66% 70% 62% benzophenone → benzopinacol → benzopinacolone triphenylmethane Answer:arrow_forward7. The reaction of methoxide anion with bromoethane to yield the ether ethyl methyl ether and the bromide anon (Br-) is an excellent example of a general reaction type called Sy2 (substitution nucleophilic bimolecular): CH,0+ CH,СH-Br a CH3-0-CH,СH; + Br- a. Change in enthalpy is -103 kJ/mol; the change in entropy is + 0.025 kJ/mol-K. Calculate DG at 300K. b. Is the reaction endergonic or exergonic? c. Is the reaction endothermic or exothermic? d. Use curved arrows to show the complete mechanism. Reaction of 2-methyl-1-butene with H-Cl could yield TWO alkyl chloride products. Draw and name 8. them.arrow_forward
- Illustrate how diazomethane and ozone can both behave as 1,3-dipoles. Indicate, by drawing their resonance forms, how they can do so and draw the products of their reaction with styrenearrow_forwardWhat reaction type is responsible for the de-colorization of bromine water exposed to cyclohexene? Group of answer choices Addition Substitution Condensation Eliminationarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY