
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Complete the peptide bond above then name the compound that is in the product in the problem.
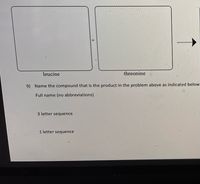
Transcribed Image Text:leucine
threonine
9) Name the compound that is the product in the problem above as indicated below
Full name (no abbreviations)
3 letter sequence
1 letter sequence
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which statement is NOT true about a peptide bond? O The peptide bond is longer than the typical carbon-nitrogen bond. CO The carbonyl oxygen and the amide hydrogen are most often in a frans configuration with respect to another. O Rotation is restricted about the peptide bond. The peptide bond has partial double-bond character.arrow_forwardWhat are the structures of the amino acids that result from the hydrolysis of all the peptide bonds in the peptide? HO 'N' HO, NH2 H COOH draw structure ... draw structure ... draw structure .. left structure middle structure right structurearrow_forwardI don't get it at all. I struggled with this question. Can you help me? Can you explain to me?arrow_forward
- Which statement is completely false below? O Amino Acid Polymers less than 50 amino acid residues long are called polypeptides O Aqueous Cysteine is a zwitterion but dry Cysteine is not O When Serine becomes aqueous, the side chain alcohol loses its Hydrogen O All are true O Amino Acids when linked in a peptide bond are called amino acid residuesarrow_forwardWhich interaction(s) is/are involved in the primary structure of a protein (choose all that apply)? a. hydrogen bonding b. salt bridge c. hydrophobic effects d. covalent peptide bondarrow_forwardDo you want to restart updates now or try toni O BIOLOGICAL MACROMOLECULES Identifying and drawing peptide bonds Highlight each peptide bond in the molecule below. In addition, list the common names of the smaller molecules that would bê released if all the peptide bonds were hydrolyzed. You can add more rows to the table if you need to. (Note: you do not need to list water.) If there are no peptide bonds in the molecule, just check the no peptide bonds box below the drawing area. H.N — CH— с—N—CH—С—NH—CH—CОО CH, CH, CH2 NH, OH no peptide bonds Explanation Check 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy Accessibil MacBook Air IIarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY