
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
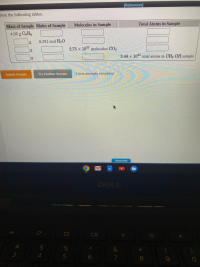
Transcribed Image Text:lete the following tables.
Molecules in Sample
Total Atoms in Sample
Mass of Sample Moles of Sample
Expert Solution
arrow_forward
Step 1
Since you have posted a question with multiple sub-parts, we will solve first three subparts for you. To get the remaining sub-part solved please repost the complete question and mention the sub-parts to be solved.
arrow_forward
Step 2
The following relations will help solve this problem.
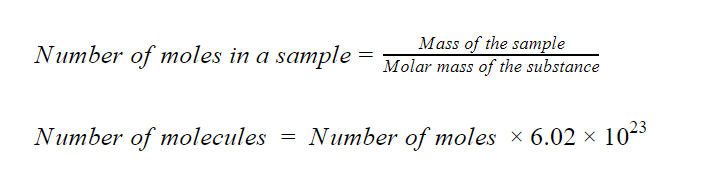
arrow_forward
Step 3
1.
4.06 g C6H6
Molar mass of C6H6 = 6(Atomic mass of C) + 6(Atomic mass of H) = 6(12) + 6(1) g/mol = 78 g/mol
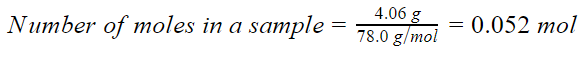
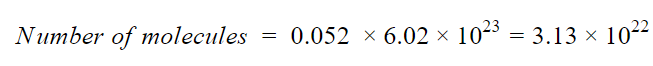
Each molecule of C6H6 contains 6 atoms of C and 6 atoms of H, thus there are total (6+6)= 12 atoms in one molecule of C6H6.
Thus,
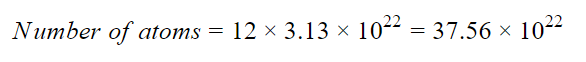
Step by stepSolved in 5 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the References to access important values if needed for this question. a. How many ATOMS of nitrogen are present in 2.50 moles of nitrogen dioxide? 1.505x10^24 atoms of nitrogen b. How many MOLES of oxygen are present in 1.08 × 10²³ molecules of nitrogen dioxide? moles of oxygen 2arrow_forwardA molar mass value is equivalent to any element'sarrow_forwardPlease convert problem nine into from representative to moles and show the steps please.arrow_forward
- To find the number of molecules in the sample of compound, which of the following values must be used in the calculation? Calculate the number of formula units present in a sample that contains 1.3 moles of potassium chloride.arrow_forwardWhich sample contains the greatest number of atoms. A sample of Ba that contains 6.72x1022 atoms or a 4.94 mole sample of Ti? The sample of contains the greatest number of atoms.arrow_forwardCalculate the mass of 400. atoms of sulfur (S)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY