
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
The correct answers are shown. Please show me the steps to calculate these specific answers.
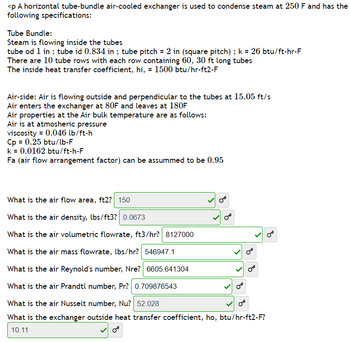
Transcribed Image Text:<p A horizontal tube-bundle air-cooled exchanger is used to condense steam at 250 F and has the
following specifications:
Tube Bundle:
Steam is flowing inside the tubes
tube od 1 in ; tube id 0.834 in ; tube pitch = 2 in (square pitch) ; k = 26 btu/ft-hr-F
There are 10 tube rows with each row containing 60, 30 ft long tubes
The inside heat transfer coefficient, hi, = 1500 btu/hr-ft2-F
Air-side: Air is flowing outside and perpendicular to the tubes at 15.05 ft/s
Air enters the exchanger at 80F and leaves at 180F
Air properties at the Air bulk temperature are as follows:
Air is at atmosheric pressure
viscosity = 0.046 lb/ft-h
Cp = 0.25 btu/lb-F
k = 0.0162 btu/ft-h-F
Fa (air flow arrangement factor) can be assummed to be 0.95
What is the air flow area, ft2? 150
What is the air density, lbs/ft3? 0.0673
What is the air volumetric flowrate, ft3/hr? 8127000
What is the air mass flowrate, lbs/hr? 546947.1
What is the air Reynold's number, Nre? 6605.641304
What is the air Prandtl number, Pr? 0.709876543
What is the air Nusselt number, Nu? 52.028
B
What is the exchanger outside heat transfer coefficient, ho, btu/hr-ft2-F?
10.11
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- As described in the Data and Results part of , all tables and graphs must have descriptive information such as titles, column or row headings, units, and axis labels, and data points must be clearly marked. All figures must be numbered and have a descriptive title. The figure number and the title should be placed below the figure. All tables must be numbered and have a descriptive title as well. However, for tables, the table number and the title should be placed above the table. In an annealing process—a process wherein materials such as glass and metal are heated to high temperatures and then cooled slowly to toughen them — thin steel plates are heated to temperatures of 900° C and thencooled in an environment with temperature of 35° C . The results of an annealing process for a thin plate is shown below. Plot the data using engineering paper incorporating the ideas discussed in this chapter.arrow_forward1. Show the step-by-step process. Do not use shortcut methods. Make it as detailed as it can be. ENCODE THE ANSWER.arrow_forwardDiscuss the relationships between the regular simplex method and the revised simplex methodarrow_forward
- top 10 practical learnings in chemical engineering that I can apply in real lifearrow_forwardUse basic formulas only. I'll upvote if it is typewritten. Thank you.arrow_forwardUsing the data below, construct a calibration curve. Include the error bars and the equation of the line and the linearity (R2) of the curve. Calcium concentration (ppm) (X-data) Absorbance (Y-data) Run 1 Absorbance (Y-data) Run 2 Absorbance (Y-data) Run 3 0.3 1109 1069 1155 1.2 1225 1168 1233 2.1 1472 1319 1389 4.2 1497 1523 1472 8.0 1833 1898 1849 16 2066 2012 2051arrow_forward
- What is a distillation-curve map?arrow_forwardUsing a bomb calorimeter, how do you measure the heat of combustion of liquid samples? Is it different with the process of measuring involved in solid samples?arrow_forwardWhat is the number of significant figures in 0.310 x 10^3. Your answerarrow_forward
- Summarize three different models for predicting the energy requirement associated with particle size reduction. Over what size ranges might each model be most appropriately applied?arrow_forwardPlease recreate this graph in a clearer version.arrow_forwardRemaining Time: 20 minutes, 12 seconds. * Question Completion Status: QUESTION 1 The dimensions of heat flux is ML2T -3 True False QUESTION 2 Dimensionless parameters are obtained using a method called dimensional analysis. O True ロFalse QUESTION 3 Click Save and Submit to save and submit. Click Save All Answers to save all answers. earcharrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The