
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:<CHE154 S20 Ch18 Sec2
Exercise 18.48 - Enhanced - with Feedback and Hints
%3D
MISSED THIS? Read Section 18.2 (Pages 788 - 799) ; Watch
KCV 18.2B, IWES 18.2, 18.3.
A 100.0-mL buffer solution is 0.175 M in HC1O and 0.150 M
in NaClO.
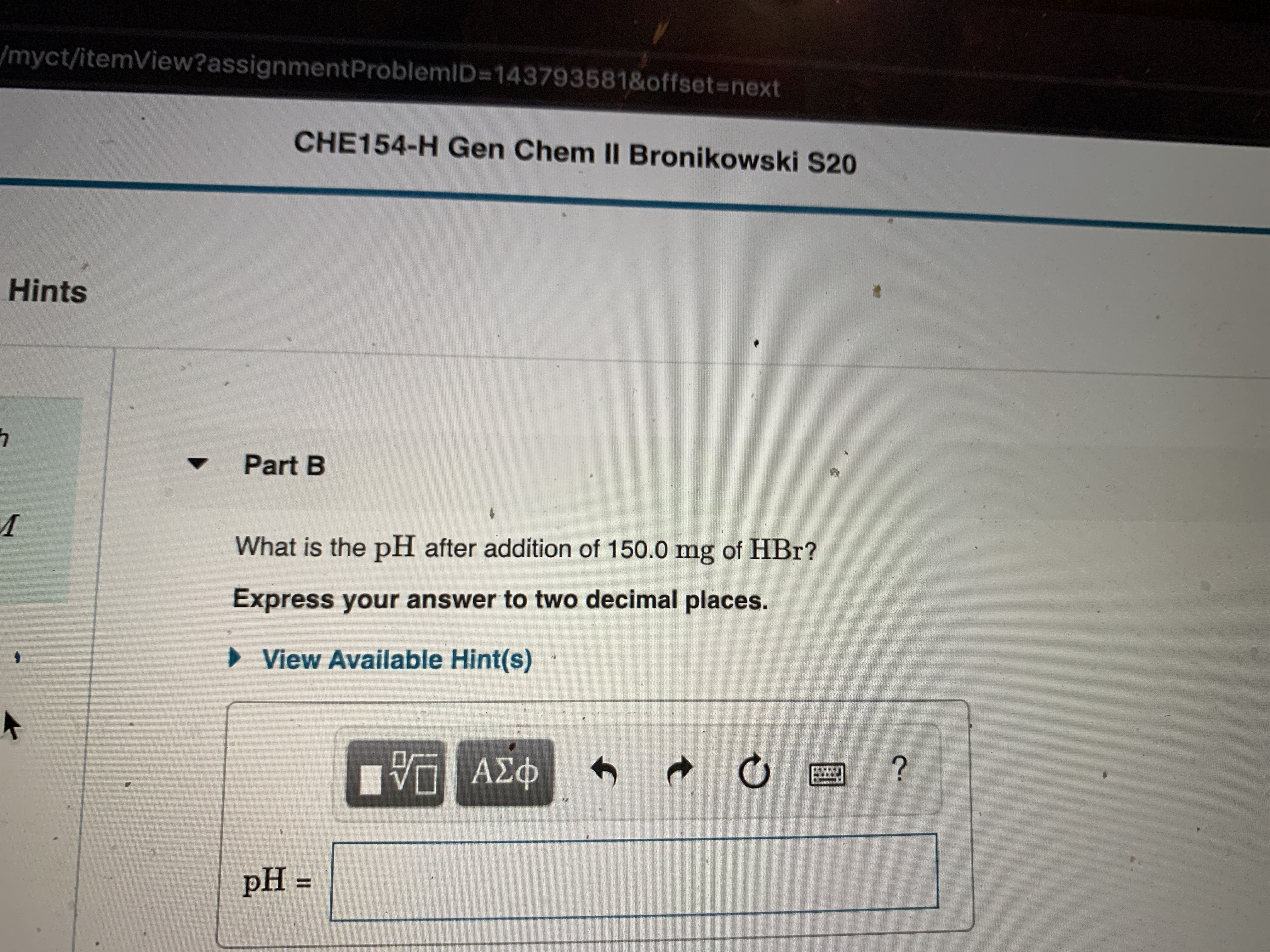
Transcribed Image Text:/myct/itemView?assignmentProblemID=143793581&offset%3Dnext
CHE154-H Gen Chem II Bronikowski S20
Hints
Part B
What is the pH after addition of 150.0 mg of HBr?
Express your answer to two decimal places.
» View Available Hint(s)
ΑΣφ
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 6 images

Knowledge Booster
Similar questions
- Consider a buffer solution that is 0.50 M in NH and0.20 M in NH4Cl. For ammonia, pKp = 4.75. Calculate the pH of 1.0 L upon addition of 0.080 mol of solid NaH to the original buffer solution.Express the pH to two decimal places.arrow_forwardPlease don't provide handwritten solution ...arrow_forwardnect ent #2 6 paae Select the single best answer. In a group 1 analysis, a student obtained a precipitate containing both AgCl and PbCl. Which of the following reagents would enable separation of AgCl(s) from PbCl2(s)? H,S Na,CO3 КОН O NH3 < Prev 4 of 5 Next MacBook Air 119arrow_forward
- When 20.0 mL of 1.0 x 10^-12 M CoCl2 are added to 20.0 mL of 6.0x10^-9 M CaS, a precipitate will____________ because _____________? a) not form, Qsp>Ksp b) not form, Qsp<Ksp c) not form, Qsp=Ksp d) form, Qsp>Ksp e) form, Qsp<Ksp Note: after finishing the problem, if not hard can you please give me a similar example of the problem?arrow_forward3. Calculate the concentration of carbonate ion in a saturated solution of CdCO3 (Ksp – 1.0 × 10–¹2). O 3.5 x 10-2M O 1.0 x 10-4M O2.7 x 10-2M O 3.5 x 10-4M O 1.0 x 10-6M X Incorrect Check out video Electrolytes for concept and Dissolution and Solubility Product Constant Part I (from time - 4:50) for examples. For an ionic compound A, By, the solubility product of a saturated solution can be found by the following equation: Ksp = [A] [B]", where [A] is the saturated concentration of ion A. 4. Check ALL of the following substances which will conduct electricity in the form given:arrow_forward123 mL of a 0.352 M Ca(NO3)2 solution is mixed with 251 mL of a 0.254 M solution of NaF at 25ºC. Calculate Qsp for the precipitate formed.arrow_forward
- Answer: yes, 0.065grams How do I find precipitate, I know it precipitates, but I don't know how to solve how many grams of precipitate there is. Solve step-by-step, on paper pleasearrow_forwardPart 1 Feedback A solution is 0.0111 Min both Brr and SO, A0.257 Msolution of lead(I) nitrate is slowly added to it with a buret. The anion will precipitate from solution first. (K, for PbBrz 6.60 x 10 K, for Pbso, - 2.53 x 10 Part 2 Feedback What is the concentration in the solution of the first anion when the second one starts to precipitate at 25°C? 011arrow_forwardPLease Help! Q: Calculate the molar solubility, s, of calcium iodatein 0.020 M Ca (NO3)2 , a completely dissociated strong electrolyte. (NO3- ion does not chemically interact with either Ca2+ or IO3-) Assume that Ksp for Ca(IO3)2= 2.0e-6 Information that I have so far... Material Balance for Calcium: [Ca2+]= s+0.020 Material Balance for Iodate: [IO3-]=2s Ksp= 2.0e-6 = [Ca2+][IO3-]2= (s+0.020)(2s)2 Thank you!!arrow_forward
- We received 15000 Ci of 28Mg, present as Mg(NO3)2 and want to dissolve is in 10 mL water at pH8. Will we form a precipitate or will the solid entirely dissolve? Only typed solutionarrow_forward< I * 日 W Chapter... G N. This webpage is using significant memory. Closing it may improve the responsiveness of your Mac. + F Supporting Materials Periodic Table Additional Materials eBook The pH of a 0.160 mM solution of the alkaloid codeine is 9.18. Determine K for codeine from these data. (Assume Kw C18H21NH₂(aq) + H₂0(I) = HC18H21 NH₂+(aq) + OH¯(aq) Submit Answer Day AD Constants and * Factors webassign.net F O Supplemental Data B L C G = 1.01 × 10-14.)arrow_forwardCalculate the solubility at 25 °C of CaF, in pure water and in a 0.0080 M NaF solution. You'll find K data in the ALEKS Data tab. 2 sp Round both of your answers to 2 significant digits. solubility in pure water: solubility in 0.0080 M NaF solution: Explanation Check Privacy Acce O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use acer oloarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY