
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
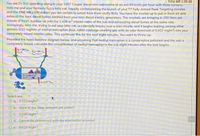
Transcribed Image Text:Time left 1:35:46
You are Dr. Evil speeding along in your 1967 Cooper diesel mini submarine at an evil 69 knots per hour with threer mors,
mini-me and your formerly fuzzy kitty cat, happily contemplating the launch of your T7 Fully-Armed Reek Targeting missiles
and the ONE MILLION dollars you are certain to extort from them stuffy Brits. You have the snorkel up to pull in fresh air and
exhaust the toxic diesel fumes emitted from your mini diesel electric generators. The snorkels are bringing in 200 liters per
minute of fresh, outdoor air into the 2.438 m interior cabin of the sub and exhausting diesel fumes at the same rate.
Annoyingly, mini-me, trying to eat your kitty cat, accidentally knocks over a mini missile, and it begins leaking, among other
gasses, 0.02 mg/min of methyl mercaptan (foul, rotten cabbage smelling gas with an odor threshold of 0.002 mg/m) into your
completely mixed interior cabin. This continues this for the next eight minutes. You want to throw up.
Provided the mass balance diagram below, and assuming that methyl mercaptan is a conservative pollutant and the sub is
completely mixed, calculate the concentration of methyl mercaptan in the sub eight minutes after the leak begins.
P-200
200
V24m/
4-00
Select one:
O o. 0.112 mg/m
O b. None of the other answers are correct
Oc 0.048 mg/m
O d. Cannot be determined with the information provided
O e. 0.028 mg/m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 11 images

Knowledge Booster
Similar questions
- According to the experiments, the change in entropy of 200 g of water when heated from an unknown temperature to 90 ⁰C at constant pressure is 250 J/K. Cp of water, assumed to be constant throughout the process, is 4.186 J/g-K. Determine the initial density of water (in kg/m³) before it was being heated. Density of water at different temperatures is presented (use this table only). Answer Format: [2 decimals]arrow_forwardA rigid insulated tank is divided into 2 equal compartments by athin rigid partition. One of the compartments contains air, assumedto be an ideal gas at 800 kPa and 300 K. The other compartment isunder a vacuum. The partition is suddenly broken and the air rushesinto the evacuated compartment. The tank pressure and temperatureeventually equilibrate. (a) define the system you will use and draw a labeled schematic.(b) write the energy balance for the system, making simplificationsas appropriate(c) what is the final temperature of the gas, K?(d) what is the final pressure, kPa?(e) To calculate the entropy change of the process, you need to definea reversible path from the initial state to the final state. What isthat path?(f) What is ∆ssys for the process.(g) To calculate the entropy change of the surroundings, you needto define a reversible path from the initial state to the final state.What is that path?(h) What is the entropy change of the surroundings. ∆ssurr(i) What is the total…arrow_forwardA pressurized tank of water has a 10-cm diameter orifice at the side bottom corner of the tank, where the water is being discharge to the atmosphere. The water level is 3 meters above the orifice. The tank air pressure above the water level is 300 kPa. Neglecting frictional effects, the initial discharge rate of water in kilograms per second (kg/s) from the tank isarrow_forward
- What constant (use the letter in your answer) does the Sherwood number have in it's definition that we need in order to perform mass transfer calculations? ANS:_________. This simplification is necessary because so many phenomena can be affecting the mass transfer at once such as geometry or convection or even temperature gradients. Basically someone else has already solved those simultaneous differential equations for that particular situation and in our practice we can use their correlation rather than rework all those details ourselves.arrow_forward2. A natural gas is flowing through a 1 m diameter and 6.5 km long pipeline (friction factor= 0.0024) at a constant temperature of 15 °C. The pressure at the inlet of the pipe is 5.7 MPa. What is the maximum mass flow rate of the gas that can be achieved and the corresponding downstream pressure? [Ans. Gmax = 1012.3 kg s-¹, Pw = 498.6 kPa]arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The