
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
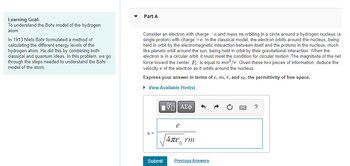
Transcribed Image Text:Learning Goal:
To understand the Bohr model of the hydrogen
atom.
In 1913 Niels Bohr formulated a method of
calculating the different energy levels of the
hydrogen atom. He did this by combining both
classical and quantum ideas. In this problem, we go
through the steps needed to understand the Bohr
model of the atom.
Part A
Consider an electron with charge -e and mass m orbiting in a circle around a hydrogen nucleus (a
single proton) with charge +e. In the classical model, the electron orbits around the nucleus, being
held in orbit by the electromagnetic interaction between itself and the protons in the nucleus, much
like planets orbit around the sun, being held in orbit by their gravitational interaction. When the
electron is in a circular orbit, it must meet the condition for circular motion: The magnitude of the net
force toward the center, F, is equal to mv²/r. Given these two pieces of information, deduce the
velocity of the electron as it orbits around the nucleus.
Express your answer in terms of e, m, r, and €0, the permittivity of free space.
► View Available Hint(s)
V=
Submit
|VE ΑΣΦ
e
|4πε, rm
Previous Answers
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The energy-level diagram of a molecule shown in (Figure 1) is irradiated by a beam of photons with an energy of 2.7 eV each. Figure Energy (eV) 3.0- 2.5- 0.7- 0- Part A What is the longest wavelength of light that the molecule can emit? Express your answer with the appropriate units. λ = Value Submit Provide Feedback A Request Answer PREZES P Units ?arrow_forwardquestion 19 helparrow_forward2. Use Max Planck's quantum theory to explain the following behaviour of photoelectrons. a. Low-intensity light does not release any photoelectrons. What will happen if the light is made brighter? Explain your reasoning. b. Low-intensity light releases photoelectrons. What will happen if the light is made brighter? Explain your reasoning. c. Low-intensity light does not release any photoelectrons. What will happen if the frequency of the light is gradually increased? Explain your reasoning.arrow_forward
- The electronic structure of a toms and molecules may be investigated using photo eletrons spectroscopy An electron in is to electron spectro mater Hoy a uniform electric field to a speed of 420kms-1 in 10µs. determine the kinetic energy of the electron Pho accelerated from nes @ 8.0x10-20 Nm 1.6x10-25 N.m 38 x 10-25 N.m d the electronic structune ota toms and maecules may be investigated using photo electron spectroscopy. An electron in a photo electron spectrometer is accelerated from rest by a uniform electric field to a speed of 420km s-lin 10,us Determine the magnitude ofthe work done on the election a b @ 160x10-25 16×10-191 490.102 50.10-201 4.0x10-2p.m L (arrow_forward4. MILLIKAN EXPERIMENT. An oil drop with a mass of 3.1 x10-1$kg absorbs 11 electrons, and then falls through an opening in two parallel, horizontal plates 1.8 cm apart. The upper plate is positive. a) Determine the charge absorbed by the oil drop. b) Determine the required potential difference between the plates in order for the oil drop to be suspended between the two plates.arrow_forward7. Determine the velocity of the electron in a positronium atom which is shown in the figure (figure is attached). Assume the radius of the positronium atom is 2 x 5.3 x 10 cm a) What is the total angular momentum about the center of the positronium atom in Problem 7? b) What is the force on the electron due to the positron? (Give both magnitude and direction.) c) What is the electric potential energy of the positronium atom?arrow_forward
- While being X-rayed, a person absorbs 3.9x10 J of energy. Part A Determine the number of 40,000-eV X-ray photons absorbed during the exam. " Templates Symbols undo rego reset keyboard shortcuts Help n= Submit Request Answerarrow_forwardSolve using GUESS method. Atomic and Nuclear Phenomena 2. The work function of a metal is 2.6 eV. What is the longest wavelength of radiation that can eject a photoelectron from this metal?arrow_forwardUsing the symmetry of the arrangement, determine the direction of the force on +q in the figure below, given that: Case I. qa=qb= +6.5 μC and qc = qd = +6.5 μC. (a) In your notebook, draw the forces on q due to qar qb²9c² (b) Due to symmetry the direction of the net force is F O qc net ✔N O qd Hint: For each force draw the x and y components. Some will add and some will cancel. (c) Calculate the magnitude of the force on the charge q, given that the square is 10.0 cm on a side and q = 2.3 μC. and 9d- Xarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON