
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Leaching of Oil from Soybeans in a Single Stage. Repeat Example 31.2-1 for single-stage leaching of oil from soybeans. The 100 kg of soybeans contains 22 wt % oil and the solvent feed is 80 kg of solvent containing 3 wt % soybean oil.
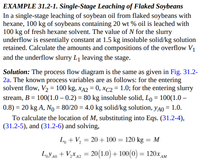
Transcribed Image Text:EXAMPLE 31.2-1. Single-Stage Leaching of Flaked Soybeans
In a single-stage leaching of soybean oil from flaked soybeans with
hexane, 100 kg of soybeans containing 20 wt % oil is leached with
100 kg of fresh hexane solvent. The value of N for the slurry
underflow is essentially constant at 1.5 kg insoluble solid/kg solution
retained. Calculate the amounts and compositions of the overflow V1
and the underflow slurry L1 leaving the stage.
Solution: The process flow diagram is the same as given in Fig. 31.2-
2a. The known process variables are as follows: for the entering
solvent flow, V2 = 100 kg, x42 = 0, XC2 = 1.0; for the entering slurry
stream, B = 100(1.0 – 0.2) = 80 kg insoluble solid, Lo = 100(1.0 –
%3D
0.8) = 20 kg A, No = 80/20 = 4.0 kg solid/kg solution, yA0 = 1.0.
To calculate the location of M, substituting into Eqs. (31.2-4),
(31.2-5), and (31.2-6) and solving,
L, + V, = 20 + 100 = 120 kg = M
%3D
LY A0 + V,*42 = 20(1.0) + 100(0) = 120x AM
Transcribed Image Text:Lo
3-
N
N vs. YA
V2-
M
N vs. XA
0.5
1.0
YA, XA
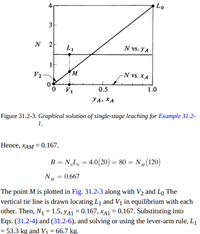
Figure 31.2-3. Graphical solution of single-stage leaching for Example 31.2-
1.
Hence, XAM = 0.167.
B = N,L, = 4.0(20) = 80 = N,(120)
N = 0.667
The point M is plotted in Fig. 31.2-3 along with V2 and Lo The
vertical tie line is drawn locating L1 and Vị in equilibrium with each
other. Then, N1 = 1.5, yA1 = 0.167, xA1 = 0.167. Substituting into
Eqs. (31.2-4) and (31.2-6), and solving or using the lever-arm rule, L1
= 53.3 kg and V = 66.7 kg.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- In microfiltration, why is an operation that combines constant-flux and constant-pressure operations used?arrow_forwardWhy must solvent be accounted for in calculating the enthalpic contribution to folding? What is the entropic contribution that solvent makes to folding? Can you please explain your answer??arrow_forwardIllustrate the preparation of porous carbon materials using sugarcane biomassarrow_forward
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The