
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
4.10. (a) For one of the compounds listed in Table B.2 of App. B, evaluate the latent heat of vaporization delta Hn by Eq. (4.13).
How does this result compare with the value listed in Table B.2?
(b) Handbook values for the latent heats of vaporization at 25C of four compounds are given in the table. For one of these, calculate delta Hn, using Eq. (4.14), and compare the result with the value given in Table B.2.
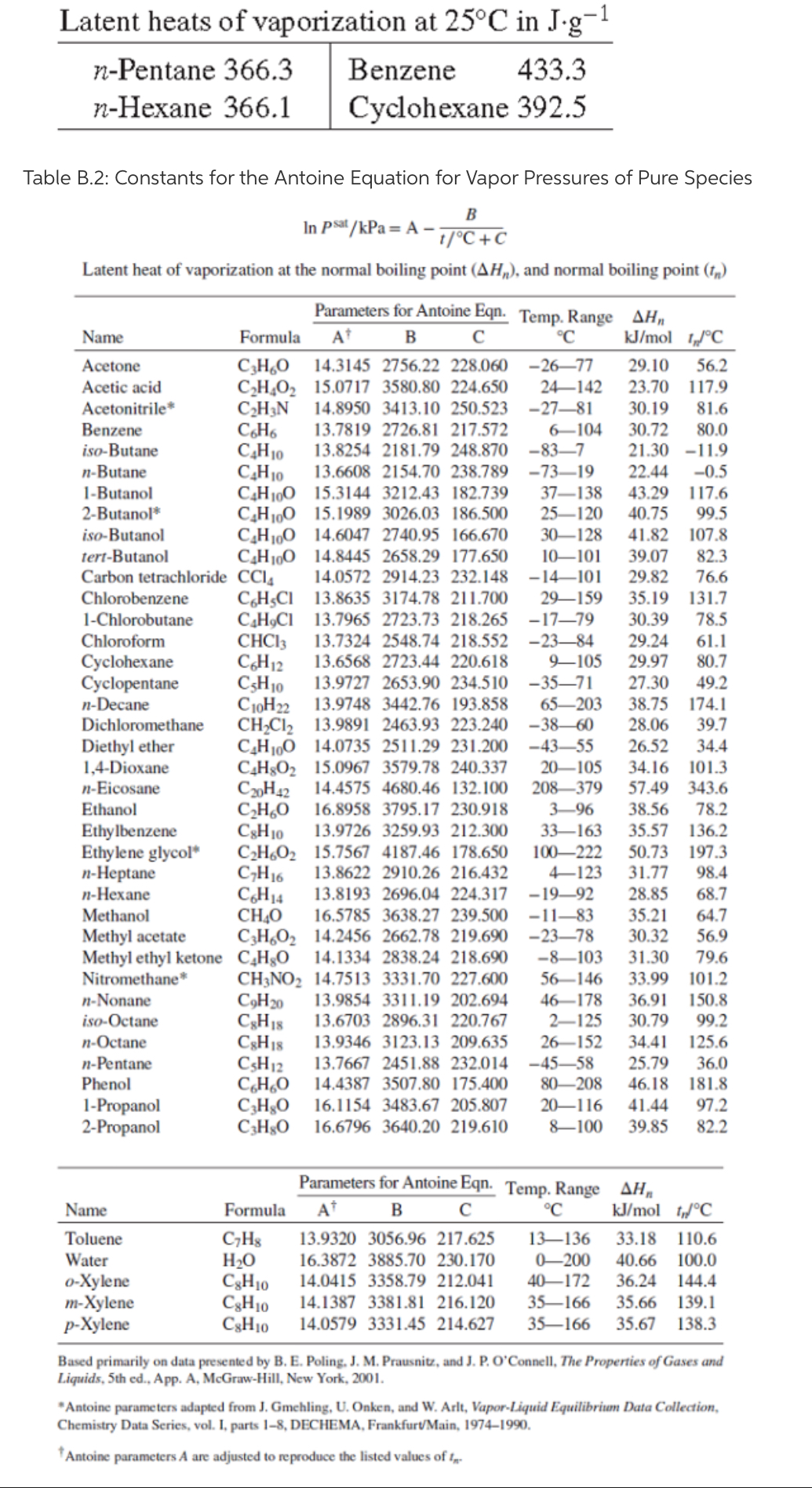
Transcribed Image Text:Latent heats of vaporization at 25°C in J-g¬1
n-Pentane 366.3
Benzene
433.3
n-Hexane 366.1
Cyclohexane 392.5
Table B.2: Constants for the Antoine Equation for Vapor Pressures of Pure Species
In Psat /kPa = A –
1/°C +C
Latent heat of vaporization at the normal boiling point (AH„), and normal boiling point (f„)
Parameters for Antoine Eqn. Temp. Range AHn
Name
Formula
At
в с
°C
kJ/mol °C
C3H̟O 14.3145 2756.22 228.060
CHO2 15.0717 3580.80 224.650
СН.N
CH6
C,H10
C,H10
C„H100 15.3144 3212.43 182.739
CH100 15.1989 3026.03 186.500
C,H100 14.6047 2740.95 166.670
C„H100 14.8445 2658.29 177.650
Acetone
-26-77
29.10
56.2
Acetic acid
Acetonitrile*
Benzene
iso-Butane
n-Butane
24–142
-27-81
6–104
13.8254 2181.79 248.870 -83-7
23.70 117.9
14.8950 3413.10 250.523
30.19
81.6
13.7819 2726.81 217.572
30.72
80.0
21.30 -11.9
13.6608 2154.70 238.789
-73–19
22.44
-0.5
1-Butanol
37-138
43.29 117.6
2-Butanol*
25-120
40.75
99.5
iso-Butanol
30–128
41.82 107.8
tert-Butanol
10–101
39.07
82.3
76.6
Carbon tetrachloride CCI4
14.0572 2914.23 232.148 -14–101
29.82
C,H§CI 13.8635 3174.78 21i1.700
C4H9CI 13.7965 2723.73 218.265 -17–79
CHCI3
CH12
C3H10
C10H22 13.9748 3442.76 193.858
CH,Cl2 13.9891 2463.93 223.240 -38–60
C,H100 14.0735 2511.29 231.200
C,H§O2 15.0967 3579.78 240.337
CH42
С-Но
С-Ню
C2HO2 15.7567 4187.46 178.650
C;H16
CH14
CH,O
C3HO2 14.2456 2662.78 219.690 -23–78
Chlorobenzene
29–159
35.19 131.7
1-Chlorobutane
Chloroform
30.39
29.24
29.97
27.30
78.5
13.7324 2548.74 218.552 -23-84
13.6568 2723.44 220.618
13.9727 2653.90 234.510 -35–71
61.1
Cyclohexane
Cyclopentane
n-Decane
Dichloromethane
9–105
80.7
49.2
38.75 174.1
39.7
65–203
28.06
Diethyl ether
1,4-Dioxane
n-Eicosane
-43-55
20–105
208–379
26.52
34.4
34.16 101.3
14.4575 4680.46 132.100
57.49 343.6
Ethanol
16.8958 3795.17 230.918
3–96
38.56
78.2
35.57 136.2
50.73 197.3
Ethylbenzene
Ethylene glycol*
п-Неptane
п-Нехane
13.9726 3259.93 212.300
33–163
100–222
13.8622 2910.26 216.432
4–123
31.77
98.4
13.8193 2696.04 224.317
-19–92
28.85
68.7
Methanol
Methyl acetate
Methyl ethyl ketone C‚HgO_14.1334 2838.24 218.690
Nitromethane*
35.21
30.32
31.30
16.5785 3638.27 239.500 -11–83
64.7
56.9
-8–103
79.6
CH3NO2 14.7513 3331.70 227.600
C9H20
C3H18
C3H18
C3H12
56-146
33.99 101.2
n-Nonane
46–178
150.8
99.2
13.9854 3311.19 202.694
36.91
iso-Octane
n-Octane
13.6703 2896.31 220.767
2-125
30.79
13.9346 3123.13 209.635
26–152
34.41
125.6
-45-58
80-208
n-Pentane
13.7667 2451.88 232.014
25.79
36.0
Phenol
14.4387 3507.80 175.400
46.18 181.8
97.2
1-Propanol
2-Propanol
С-Н,О
C3HgO 16.6796 3640.20 219.610
16.1154 3483.67 205.807
20–116
41.44
8-100
39.85
82.2
Parameters for Antoine Eqn. Temp. Range
At
дн,
kJ/mol t°C
Name
Formula
°C
Toluene
33.18
С-Нs
Н-о
C3H10
C3H10
C3H10
13.9320 3056.96 217.625
13–136
110.6
Water
16.3872 3885.70 230.170
0-200
40-172
35-166
40.66
100.0
36.24
o-Xylene
m-Xylene
p-Xylene
14.0415 3358.79 212.041
144.4
14.1387 3381.81 216.120
35.66
139.1
14.0579 3331.45 214.627
35–166
35.67
138.3
Based primarily on data presented by B. E. Poling. J. M. Prausnitz, and J. P. O'Connell, The Properties of Gases and
Liquids, 5th ed., App. A, McGraw-Hill, New York, 2001.
*Antoine parameters adapted from J. Gmehling, U. Onken, and W. Arlt, Vapor-Liquid Equilibrium Data Collection,
Chemistry Data Series, vol. I, parts 1-8, DECHEMA, Frankfurt/Main, 1974–1990.
TAntoine parameters A are adjusted to reproduce the listed values of tg-
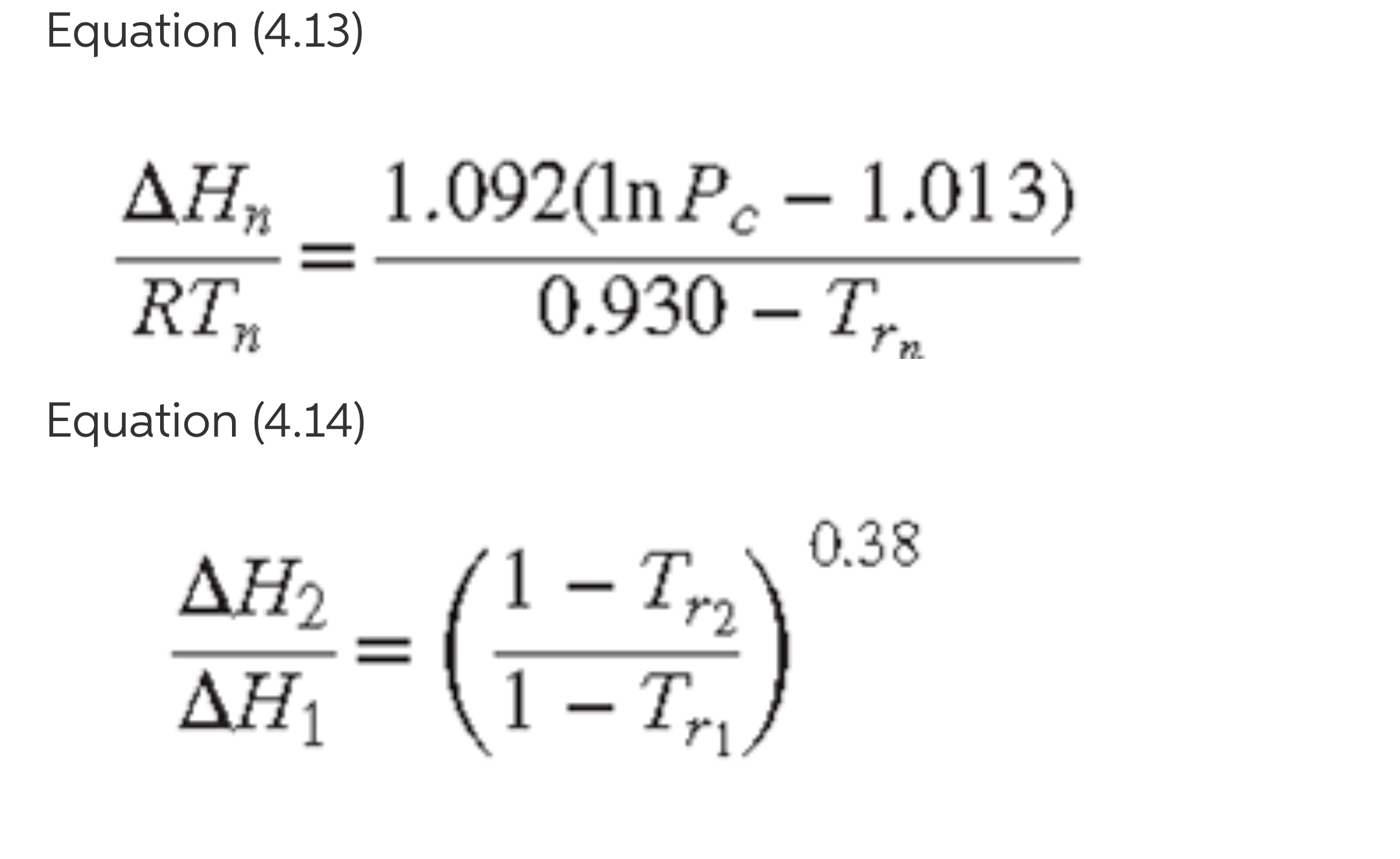
Transcribed Image Text:Equation (4.13)
ДН, 1.092(n Р. - 1.013)
0.930 – T,.
RT,
Equation (4.14)
0.38
ΔΗ
1 – Tr2
Tyg
ΔΗ1
1 – Tr,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 8 steps with 12 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A food product containing 80% moisture content is being frozen. Estimate the specific heat of the product at -8 ° C when 82% of the water is frozen. The specific heat of the dry product is 2.5 kJ / (kg ° C). it is assumed that the specific heat of water at -10 ° C is the same as the specific heat of water at 0 ° C, and the specific heat of ice follows the function Cp es = 0.0062 Tbeku + 2.0649. Cp frozen product = AnswerkJ / kg ° C.arrow_forwardcalculate the heat required to raise the temperature of 1 mol of methane from 260 to 600 °c in steady flow process at a pressure sufficient low that methane may be considered an ideal gasarrow_forwardA food product containing 80% moisture content is being frozen. Estimate the specific heat of the product at -6 ° C when 80% of the water is frozen. The specific heat of the dry product is 2 kJ / (kg ° C). It is assumed that the specific heat of water at -10 ° C is the same as the specific heat of water at 0 ° C, and that the specific heat of ice follows the function Cp es = 0.0062 T frozen + 2.0649. Cp frozen product = kJ / kg ° C.arrow_forward
- Handwritten Only.arrow_forwardFor substance A, it is known that the vapor pressure at 300K is 100kPa. It is also known that the boiling point temperature is 340K at 150 kPa. Other given information about substance A: Cpl= 200 J/mol.K and Cpv = 180 J/mol.K c) Using the correlation developed in part a), determine the latent heat of vaporization of substance A in kJ/kmol d) 2 mol of substance A goes through a process where its temperature changes from 400K to 300K at constant pressure 150kPa. i) What phase is present initially? ii) What phase is present at the final state? iii) Determine the total amount of energy transferred (in kJ) through the process and state whether the energy is transferred to or from the substance.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The