
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
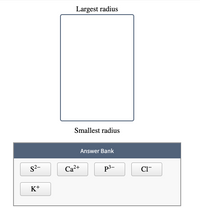
Transcribed Image Text:### Ion Size Order
**Objective:**
Arrange the given ions in order of their ionic radii, from the largest to the smallest.
**Instructions:**
Drag and drop each ion from the Answer Bank into the designated box, ordering them by their radius from top (largest radius) to bottom (smallest radius).
**Diagram Explanation:**
- The central box vertically lists the ions to be arranged.
- The top is labeled "Largest radius", and the bottom is labeled "Smallest radius."
**Answer Bank:**
- \( \text{S}^{2-} \)
- \( \text{Ca}^{2+} \)
- \( \text{P}^{3-} \)
- \( \text{Cl}^- \)
- \( \text{K}^+ \)
Use your knowledge of ionic radii to correctly arrange the ions. This interactive exercise is designed to help you understand trends in ionic sizes across different ions in the periodic table.
### Concepts to Consider:
- Ionic radii of anions (negatively charged ions) can be larger than their respective neutral atoms due to electron-electron repulsion when electrons are added.
- Ionic radii of cations (positively charged ions) tend to be smaller than their respective neutral atoms due to the loss of electrons and subsequent reduction in electron-electron repulsion.
- Trends in ionic radii can be influenced by the charge and electron configuration of the ions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- mass of copper = 2.494g; Wavelength = 830.6 Volume of volumetric flask - 50 ml Here's the absorbance of all the test tubearrow_forwardDI Chapter 2 Multiple Choice Question 69 Part A What is the frequency of light with a wavelength of 0.440 um? O 2.55 x 1013 sec1 O 5.93 x 1011 sec1 O 6.81 x 1014 sec O 7.28 x 1014 sec1 O 6.81 x 1011 sec1 Submit Request Answer Provide Feedback P Type here to search ? f2 f3 f4 esc 15arrow_forwardThe mean radius of an orbital on the hydrogen atom is given by the expression Pu-(1-3 (1-10 + 1)) n/a (1+3(1) Z Calculate the mean radius of the 2p orbital. Select one O a 6 a b. (25/2) a c5a, Od (27/2) aarrow_forward
- Pb(NO3)2(aq) + KOH(aq) = 1. what is the balanced equation 2. what is the ionic equation 3. what is the net ionic equationarrow_forwardPart A Place the following elements in order of decreasing atomic size: silicon, nitrogen, helium, potassium, magnesium, and carbon. Rank from largest to smallest. To rank items as equivalent, overlap them. Reset Help K Mg SiCN He Largest Smallest O The correct ranking cannot be determined. 37°F Mostly cloudy F7 F8 F9 F10 F11 F12 PrtSc Pause Ins Del A Scr Lk SysRq Break & + Backspacearrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- Chrome File Edit View History Bookmarks Profiles Tab Window Help 31% Fri 10:35 PM sciencenotes.org 101 Chem101 Identify the region o x b My Questions | bart X CHEM 111-Ch 3 Flas X + 1 18 Update : IA VIIIA app.101edu.co 1A 8A Periodic Table of the Elements -1, +1 Apps M Gmail" YouTube Translate P MyLabsPlus | Pear... TE MYTCC Blcackboard Reading List H Не >> 2 13 IIIA ЗА 14 15 16 17 IIA IVA VA VIA VIIA Hydrogen 1.008 Atomic Number Helium 4.003 Valence 2A 4A 6A 5,-3,-3 8 5A 7A 4 5 +4,+3,+2,+1 -110 +1 +2 +3 3 7 Symbol 4,-3 -2,-1 Question 18 of 50 Submit Li Ве F Ne Lithium 6.941 Beryllium 9.012 Carbon Oxygen 15.999 Boron Nitrogen 14.007 Fluorine 18.998 Neon 20.180 Name Atomic Mass +4,-4 15 *5,*3,-3 16*6,+4,+2,-217*7.+5,+3,1 18 +1 +2 13 +3 11 12 14 Na Mg AI Si ci| Ar 3 IIIB ЗВ 4 IVB 4B 6 VIB 6B 7 VIIB 7B 8 10 Identify the region of the electromagnetic spectrum where the wavelength VB 5B VI 8 11 IB 1B 12 IB 2B Magnesium 24.305 Silicon 28.086 Sulfur 32.066 Chlorine 35.453 Argon 39.948…arrow_forwardCan you explain it? How to find the answer?arrow_forwardPart A Rb+ Spell out the full name of the ion. Submit Request Answerarrow_forward
- Explain the meaning of the following terms and phrases: 1.1 Precision of a set of results. 1.2 Noise around an analytical signal. 1.3 A shelf-life of an aqueous trace metal standardarrow_forward140.1 140.9 144.2 (145) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 17 Actinide 90 91 92 93 94 95 96 97 98 99 100 101 102 10 Series Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No 232.0 231.0 238.0 (237) (244) (243) (247) (247) (251) (252) (257) (258) (259) (26 Question 4 The Bohr model of the atom assumes that which of the following quantities for the electron in a hydrogen atom is/are quantized? i. Speed ii. Direction of spin axis ii. Energy iv. Radius of its orbit iii and iv O i and iii O i only O i, ii, and i O i, ii, and iv Next • Previous MacBook Pro Q Search Bing 23 & 7 8. OCarrow_forwardGet Homework Help G how many pre mm Evaluate Extra Info The volume for the sheet of foil is incorrect. 10) Dimensional Analysis Incomplete The closest star, other than the sun, to earth is Alpha Centauri, which is 2.57 x 1013 miles from earth. The farthest star that can be seen with the naked eye is V762 Cas which is 9.57 x 1016 miles from earth. Suppose you discovered a star that was 6.45 x 1016 miles from earth. If the speed of light is 2.999 x 10° m/s, how many years would it take light from the star to reach earth? Time for light to reach earth years Evaluate Check your answer Complete! 11) Dimensional Analysis One atom of gold weighs 3.27 x 1013 ng. How many gold atoms are in 1.0 g of gold?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY