
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
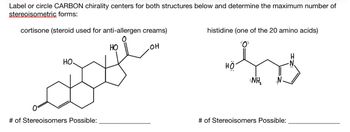
Transcribed Image Text:Label or circle CARBON chirality centers for both structures below and determine the maximum number of
stereoisometric forms:
cortisone (steroid used for anti-allergen creams)
HO
.OH
HO
Boye
# of Stereoisomers Possible:
histidine (one of the 20 amino acids)
HO
NH₂
# of Stereoisomers Possible:
Expert Solution
arrow_forward
Step 1: Formula Used
We know if n is the number of chiral carbon atom that means the carbon atom containing 4 different groups .
Then number of stereoisomers = 2n
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the stereochemical relationship between the following pairs of molecules? In the blank type either: same, enantiomers, or diastereomers CH3 H Br Br CH₂CH3 H HO H CH3 Br CH3 CH3 HO- CH3 B A:B D:E OH blank 1 blank 4 A:C D:F с CH3 blank 2 blank 5 CI D H B:C E:F CI E H H- blank 3 blank 6 -H CI CH3 Farrow_forwardDraw the most stable chair conformation of limonene. Label all the nonequivalent protons in the cyclohexene ring with an asterisk (*). The natural product drawn below is eugenol, derived from cloves, nutmeg, bay leaf, and cinnamon. Predict the 8 values for the 1H NMR for each of the protons. H3CO Ha Hb Hc HO Draw a splitting tree for Ha and Hbarrow_forwardGiven the following structure and numbering: H CO,H 3 H3C 1 A (a) Label the stereocenters of A as R or S and draw a Fischer Projection of the enantiomer of A with C1 on top and C4 on bottom. (b) Draw a Newman Projection of a diastereomer of 4 looking down the C2-C3 bond with C2 in front.arrow_forward
- Hw 1.arrow_forwardPlease send me the question in 20 minutes it's very urgent plzarrow_forwardConsider this molecule, which contains one chirality center. What is the highest-priority substituent at this chirality center? () -CH,CH, -CH, -Br What is the lowest-priority substituent at this chirality center? -CH, -CH₂CH₂ Name the structure. (S)-2-bromobutane (R)-2-bromobutane 0 FOREX OH zoom in Br Qarrow_forward
- Can you please explain how to go about assigning chirality priority from 1-4?arrow_forwardWhat is the stereochemical relationship between the compounds below? OH HO HOʻ H OH Но- Но H- H3C- H3C- -OH Но CH3 ČH3 CH3 ČH3 I II III O l and III are identical and are diastereomers of II O Il and IIII are enantiomers and are diastereomers of I O Il and III are identical, and are enantiomers of I O l and Il are enantiomers, and are diastereomers of II O l and Il are enantiomers and III is a meso compoundarrow_forwardHow to ID and assign each stereocenter (using cahn, ingold) R, S system.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY