
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Label each of the following structure as a reducing or nonreducing carbohydrate.
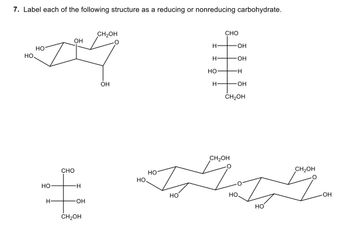
Transcribed Image Text:7. Label each of the following structure as a reducing or nonreducing carbohydrate.
CH2OH
ОН
но
та
но.
но
Н
CHO
-Н
ОН
CH₂OH
ОН
НО,
но
НО
Н
H
НО
H
CHO
-OH
CH2OH
OH
∙H
ОН
CH2OH
гдд
НО.
НО
CH2OH
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name this structure found in carbohydrates. OR R-C-R |arrow_forwardWhat type of glycosidic bond would be formed between the two carbons circled in the molecules below? CH₂OH OH OH OH OH a 1,4 glycosidic bond a 1, 5 glycosidic bond 0 B 2.6 glycosidic bond B2.5 glycosidic bond + CH₂OH OH OH OH OHarrow_forwardIn solution, glucose is mostly found in the cyclic hemiacetal state, which lacks an aldehyde group. How are mild oxidising agents able to oxidise glucose?arrow_forward
- Mark the TRUE alternative regarding the following trisaccharide: a) It has two glycosidic bonds. b) It has reducing power. c) All the monosaccharides that constitute it are glucose units. d) It has three glycosidic bonds. e) It presents mutarotation.arrow_forward14. Label the glycosidic bond. Is this a reducing sugar? 6 CH₂OH 1 CH₂OH 2 5 4 3 OH OH LO 5 OH OH 1 4 OH 3 OHarrow_forwardHow much lactic acid (C3H6O3) is produced when 225g glucose (C6H12O6) is used as a substrate in fermentation. Assuming that the sugar is completely fermented to lactic acid.arrow_forward
- Identify which of the following chemical formulae does not represent carbohydrate. Explain your answer. C6H12O6 C5H12O5 C4H8O5arrow_forwardWhich is the basic unit from where esters/ biodiesel are produced? unsaturated chains disaccharide Triglyceride Alpha- amino acid Polysaccharidearrow_forwardProvide structures for the reactants, intermediates, or products, as indicated, in the following reactions. Draw the structures in the boxes provided. SOC1₂2 pyridinearrow_forward
- Polyhydroxylated ketones and aldehydes, such as the aldopentoses and the ketopentoses, are also known as which of the following? O carbohydrates O polyhydroxylic acids O proteins O salts O alkenes O fatty acids O polyketonesarrow_forwardWhat is the structural relationship between glucose and galactose?arrow_forwardShow how the structure of glycogen and cellulose, is related to its function in each case?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY