
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Is it correct
Transcribed Image Text:4:39 PM Mon 13 Nov
Q
ch
X Fnb Lab report 1
no
第2頁,共5頁
X
*
Ch 8 Chemical.. x
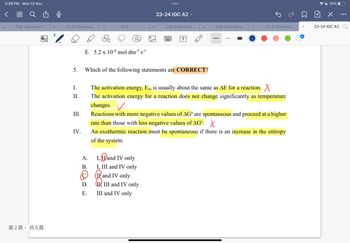
5.
I.
II.
III.
IV.
E. 5.2 x 10-4 mol dm-³ s-1
Ch3
A.
B.
D.
E.
23-24 IGC A2 ✓
I, I and IV only
I, III and IV only
249 4 Kinetics..
and IV only
II, III and IV only
III and IV only
*
Which of the following statements are CORRECT?
T
X
8:0
249 4 Kinetics... X
n
The activation energy, Ea, is usually about the same as AE for a reaction. X
The activation energy for a reaction does not change significantly as temperature
changes. ✓
Reactions with more negative values of AG are spontaneous and proceed at a higher
rate than those with less negative values of AG. X
An exothermic reaction must be spontaneous if there is an increase
of the system.
Ch 8 Chemical..
the entropy
31%
dan
23-24 IGC A2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2A + b <-> C. Kc = 9.1C + D <-> 2E. Kc = 3.2 calculate Kc for: A + 1/2B + 1/2D <-> Earrow_forwarda laccd sign i X C tab McGraw-Hil X A ALEKS-Shu X https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym9HtY-pEBmXkvCfq... O CHEMICAL REACTIONS Calculating ion molarity using solute mass shift ↑ Calculate the molarity of Cl anions in the chemist's solution. Be sure your answer is rounded to the correct number of significant digits. caps lock esc Explanation ALEKS A chemist prepares a solution of vanadium(III) chloride (VC13) by measuring out 1.50 g of VCl₂ into a 300. mL volumetric flask and filling to the mark with distilled water. ILION K →1 E mol L ? Type here to search A Graw X HI Check 12 (8) 2 Z W S # 3 X alt $ D 4 C x10 f5 % X 100 LO 5 V f6 4- (0) 6 ALEKS G 14.1 f7 4+ & Y B hp 7 X H McGraw-Hi x A ALEKS-Shix Sign In 시기 fg IAA * © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibilit # a @ 76°F ㅅ 11:03 7/25/2 00 8 N 19 DII 9 fo K M DDI 2/5 0 P 12 insert [ = X + alt prt sc 46 Shusha 0 1 A backs paus ctearrow_forwardQ. 9 is the one I need help witharrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY