
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:لا
app.aktiv.com
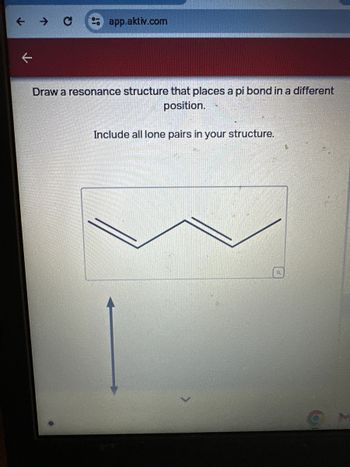
Draw a resonance structure that places a pi bond in a different
position.
Include all lone pairs in your structure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Draw a resonance structure that places a pi bond in a different position. Include all lone pairs in your structure. teen I 0:0 Drawingarrow_forwardDraw a resonance structure that places a pi bond in a different position. Include all lone pairs in your structure. Drawing HVO Qarrow_forwardDraw an equivalent resonance structure that minimizes charge. Include all lone pairs in your structure. OU Harrow_forward
- 3. One curved arrow shows the movement of an electron pair. o a bond (sigma or pi) involves two electrons, so each curved arrow indicates some change in bonding. Step 6: When a curved arrow starts from a o bond and points to a distant atom, the o bond breaks and a new o bond is formed. A -B A-C B* B-C A* or Note that two possible cations can be formed, depending on whether the electrons from the o bond move with atom A or B. Draw curved arrows to form the products shown. Select Draw Rings More Erase Al H. -0: + AIH, H - Al -H étv 0 hulu MacBook Pro GSearch or type URL & 5 7 T Y * 00arrow_forwardHydrogen cyanide, HCN, is a highly poisonous compound that vaporizes slightly above room temperature. a) How many valence electrons does C have? b) How many valence electrons does H have? c) How many valence electrons does N have? d) How many total valence electrons does the HCN molecule have? e) Draw the Lewis structure for HCN that minimizes the formal charges on all atoms. f) How many electron domains are on the central atom? g) What is the molecular geometry of HCN? h) Is the molecule polar?arrow_forward3. The following shows all resonance structures for the following molecule. a. Draw in all implied lone pairs. b. Draw in curved arrows that show the flow of electrons, making sure the arrows show the precise starting point and destination of the electrons. Label each arrow as: lp →→→л (p=lone pair) c. d. Rank the resonance structures from most stable to least based on the number of formal charges and atoms that lack an octet of electrons. ol-of-o. B A D d-d-d-o E C F Garrow_forward
- Please provide only typed answer solution no handwritten solution neededarrow_forwardWhich of the following species is a valid resonance structure of A? Use curved arrows to show how A is converted to any valid resonance structure. When a compound is not a valid resonance structure of A, explain why not.arrow_forwardDraw the Lewis structure of chlorine pentafluoride. Identify BOTH its electron pair and molecular geometries. If an overall dipole moment is present, draw the arrow.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY