
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
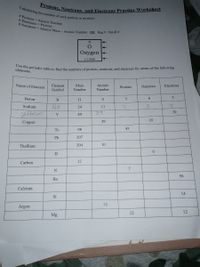
Transcribed Image Text:Use the periodic table to find the numbers of protons, neutrons, and electrons for atoms of the following
Calculating the number of each particle in an atom:
Protons, Neutrons, and Electrons Practice Worksheet
# Neutrons = Atomic Mass - Atomic Number OR Big # - Small #
# Protons = Atomic Number
# Electrons = Protons
8.
Oxygen
15.999
elements.
Name of Element
Atomic
Element
Mass
Protons
Neutrons
Electrons
Symbol
Number
Number
Boron
B
11
6
Na
Sodium
24
11
Hrium
39
39
Y
89
Сopper
29
35
Tc
98
43
Pb
207
Thallium
204
81
H
12
Carbon
7
N
56
Ba
Calcium
14
Si
18
Argon
12
12
Mg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Short answer please Maximum 50 words Thank youarrow_forwardPractice #3 2# 2# Element Symbol Charge +, 0,- Protons Neutrons electrons Carbon-13 Hydrogen-2 1 He 24 - Nitrogen 7 7 16 Охудеn 8 0.8 11 12 12 List any ions of an element from the table List any isotopes of an element from the table 6p 4e 11p 12n 13e 7n 7e 13+ 14 n Ions? How many different elements are pictured above? acerarrow_forwardQuestion 8 Enter correct values for each of the following. Symbol Atomic Number Mass Number # of Protons # of Neutrons # of Electrons 5¹Cr3+ R F B F Barrow_forward
- calculate subatomic particle number 8 15 17 O P CI 16 31 35 protons = protons= protons= neutrons= neutrons= neutron= electrons= electrons= electrons=arrow_forwardb) Develop a relationship (in the form of a single sentence or math equation) that car the charge of an atom or ion. 6. Play with the simulation to discover what affects the mass number of your atom or ion a) What is a rule for determining the mass number of an atom or ion? Practice applying your understanding by playing 1" and 2d levels on the game screen.arrow_forwardWhat is the mass # of an ion with 107 electrons. 158 neutrons, and a +1 charged? Express answer as an integerarrow_forward
- How many electrons in Ag-107?arrow_forwardHow many 126 electrons 82 electrons 78 electrons 86 electrons Question 3 How many protons, electrons and neutrons does Cl1 ? 17 protons, 18 electrons, 18 neutrons 18 protons, 17 electrons, 35 neutrons Сopy Share Select all Web search trons O 18 protons, 19 electrons, 35 neutrons Question 4arrow_forwardQ17arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY