
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
(1) Identify nucleophile and electrophile
(2) Draw mechanistic arrows
(3) Identify which elementary step is occurring
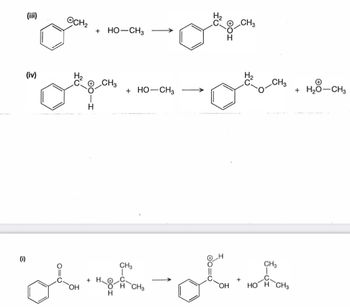
Transcribed Image Text:(iii)
(iv)
CH₂
H
+ HO-CH3
CH3
+ H₂O
H
+ HO-
-CH3
(i)
"oh ghat
OH
H CH3
CH3
CH3
Culgen
+
CH3
OH HOH CH3
+ H₂O-CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Of the following halonium (bromonium) ions; Indicate for each one which of the two carbons is more susceptible to being attacked by the nucleophile and explain why. Br+ Br* Br Br X X CH3 CH3 H CH3 H |||4 H H3C... H 114 H Harrow_forwardConsider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply? Is first order in haloalkane and first order in nucleophilearrow_forwardDraw the initiation, propagation, and one termination step for the radical bromination of 2- methylpropane with electron arrows.arrow_forward
- For each pair of reactions (a) - (d), indicate which is faster. a) A CH₂CH₂Br or B CF₂CH₂Br b) A CH₂CH₂Br or B CH₂CH₂Br c) A B d) A or B In (a ✓ [Select] B In (b A In (c). [Select] in (d), [Select] + CHÍNH, CHÍNH, CHÍNH, CFJNH, -0 EtOH V DMF is faster. ✓is faster. is faster. is faster. "Br CHÍNH CHỊCH "Br CHÍNH,CH, CFS Br CHÍNH CH, CHI Br CENH, CH, CH + HO + HO но + ECCOarrow_forwardIdentify the electrophile in the following electrophilic addition reaction step. Explain your choice.arrow_forwardDraw the mechanism for the following reactions. i. [PtCla]? + NH3 → [PtCl3(NH3)] +Cl¯ (Association mechanism) ii. [Co(NH3)5Cl]2+ + H2O → [Co(NH3)5H2O]3+ + Cl (Dissociation mechanism)arrow_forward
- 3) Any compound or ion with at least one lone pair can be considered a base and/or a nucleophile. The relative basicity and nucleophilicity of the species is one of the most important factors that determine the mechanism of a reaction on sp³-carbon atoms. Please show an example of a) a reagent that is a strong nucleophile and a strong base, b) a reagent that is a weak nucleophile and a strong base, c) a reagent that is a strong nucleophile and a weak base, d) a reagent that is a weak nucleophile and a weak base. Which nitrogen atom in the structure below is most nucleophilic? Please explain by discussing the electron density around each nitrogen atom. Show at least three resonance structures of the compound. N.arrow_forwardUsing drop down answer the questions which correctly characterizes the mechanism of the reaction shown? CH3SNA, CH3OH SCH3 Br # of Transition States? = choose your answer... # of Intermediates? = choose your answer... Rate Determining Step?= choose your answer...arrow_forwardRefer to the reaction energy diagram to answer parts (a) through (e). (a) What is the rate-limiting STEP? (Select from 1, 2, 3, or 4) (b) The TRANSITION STATE for the fastest step is: (c) In the 3rd step which INTERMEDIATE structurally resembles the transition state according to the Hammon postulate? (d) Which STEP NUMBER is endergonic? (e) The number of intermediates in the overall reaction is:arrow_forward
- Draw both the organic and inorganic intermediate species. Include nonbonding electrons and charges, where applicable. Include hydrogen atoms.arrow_forwardWhat happens to the rate of an Sx2 reaction when both [RX] and [:Nu ] are halved? The rate (select) by a factor ofarrow_forwardSelect the option that is a propagation step for the following eaction. Br+ Br Br + HBr + HBr Peroxides Br-Br Peroxides Br + Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY