
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:Isonitriles (A) are an important class of compounds because of the versatile reactivity of the functional group, enabling the
preparation of numerous new compounds and natural products. Isonitriles can be converted to isonitrile dihalides (B), which
represents a useful procedure for temporarily hiding the reactivity of an isonitrile (Tetrahedron Lett. 2012, 53, 4536-4537).
H,C-C-NEC:
H,C-c-N=cc,
A
B
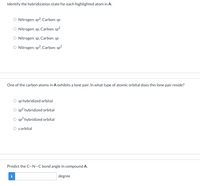
Transcribed Image Text:Identify the hybridization state for each highlighted atom in A.
Nitrogen: sp?, Carbon: sp
Nitrogen: sp, Carbon: sp?
O Nitrogen: sp, Carbon: sp
O Nitrogen: sp?, Carbon: sp?
One of the carbon atoms in A exhibits a lone pair. In what type of atomic orbital does this lone pair reside?
sp hybridized orbital
sp? hybridized orbital
O sp hybridized orbital
O sorbital
Predict the C-N-C bond angle in compound A.
i
degree
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If 3.5L of a 4.8 mol/L SrCl2 solution is diluted to 45L, what is the molarity of the diluted solution? Write/Balance the following chemical equations: a) Co(NO3)s ( aq) + (NH4)2S ( aq) Co2S3(s) NH.NO:(aq) b) Solid iron (III) oxide reacts with hydrogen gas to form solid iron and liquid water.arrow_forwardParagraph Styles Edi 7. Answer ALL parts of the question (a) Predict the organic products formed when 3-methoxybenzaldehyde is heated with formaldehyde in the presence of concentrated sodium hydroxide (after final acidification with HCI) (Scheme Q7a): 1. NaOH, heat MeO, H. H. 2. HCI Aromatic (Scheme Q7a) (b) Give the full name of this reaction. (c) (1) Identify the electrophile in the hydride transfer step to form the aromatic product. (ii) Draw a mechanism for the reduction of this electrophile.arrow_forwardCan someone please help me out with this? It has to have Hyaluronic Acid, (C14H21NO11)n, as either the product or the starting material. Thanks in advance!arrow_forward
- te Bb Welcome, Kawtha... Maps News Home GE [Review Topics] [References] 1. Br H,SO, /H,O H20 Mg2+ Br HSO, 2. OH Na HCO, O Na CO2 H20 h = Sy1 Nucleophilic substitution i= SN2 Nucleophilic substitution- j= Electrophilic aromatic substitution a = Proton transfer e = Electrophilic addition b = Lewis acid/base f= El Elimination c = Radical chain substitution d = Radical chain addition g = E2 Elimination Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - j for your answers. 2. Submit Answer Retry Entire Group 9 more group attempts remaining Previous Next Email Instructor Save and Exi Cengage Learning | Cengage Technical Supportarrow_forward9. Compound X, C25H41 Br3, reacts with excess H2/Pd to give a C25H47 Br3 compound. How many rings are in X? How many double bonds are in X? Show your work.arrow_forwardCHEM-240-x1-x2- X 09 KEY Stereoche X *Organic Chemist x functional groups X 08 KEY Constitutic X *Molecular Model X b Draw the lowest Course: NEUR-35 X A https://moodle.viterbo.edu/pluginfile.php/1286283/mod_resource/content/0/Molecular%20Models%20Lab%20assignment%202.. Not syncing A) Read aloud 7 Draw E Highlight O Erase 3 of 4 5. Draw (15,3R)-1-bromo-3-methylcyclohexane in its lowest energy conformation. 6. а. Draw the following molecule as a flat ring skeletal structure with correct stereochemistry. H b. Is the chair in 6a. in its lowest energy conformation? Provide your rationale. 11:31 AM O Type here to search 9. 10/5/2020 +arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY