
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
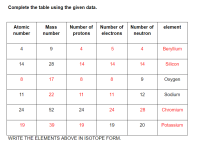
Is this correct? refer to the red numbers and words. Thanks in advance!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- solve by filling in blanks like it asks pleasearrow_forwardA sample of water is found to contain 0.012 ppm of Pb2* ions. Calculate the mass of lead ions per liter of this solution (assume the density of the water solution is 1.0 g/mL). O 5.9x10 g/L Pb2 O 1.2*10 g/L Pb O 1.2*10 11 g/L Pb2 O 1.2%10 5 g/L Pb2+ Activate Windows Go to Settings to activate Windo inspiron F10 F11 Backspac P F K Ente B Shift Alt Alt Ctrlarrow_forward1a. Throughout the experiment, what are some possible sources of error that could have led to someone recovering a significantly smaller amount of table salt (NaCI) than was originally present in the sample mixture. 16. What are some possible sources of error that could have led to someone to appear to have recovered a significantly larger amount of sand (SiO2) than was originally present in the sample mixture.arrow_forward
- Please don't provide handwritten solution ...arrow_forwardThe percent concentration of a solution is a ratio of the amounts of dissolved solute and solution, expressed as a percentage. The percent concentration of a solution can be written generally as % concentration = amount of solute x 100% amount of solution Depending on the situation, a percent concentration might be calculated for mass/mass, volume/volume, or mass/volume. tab os lock ntrol esc alt option Q A Z 5,566 2 W S AUG 2 F2 X H command 3 20 E F3 D $ 4 C 888 R Part A F4 70 Calculate the mass percent of a solution that is prepared by adding 54.8 g of NaOH to 461 g of H₂O. Express your answer numerically. ► View Available Hint(s) LIVE ΑΣΦΑ++ ο Submit Part B F [ΫΠ| ΑΣΦ Calculate the mass/volume percent of a NaCl solution in which 136 g of NaCl is dissolved in enough water to give a total volume of 1.44 L . Express your answer numerically. View Available Hint(s) % 5 V T G - 0 B MacBook Air F6 Y & - V 8⁰ 7 H #tv NA ? F7 ? U N %(m/m) %(m/v) 00* J FB M ( 9 K L F10 P command Review I Cons…arrow_forwardPlease answer in tipping format forarrow_forward
- Q22: Phosphate buffer solution is purchased as a(n) 2x stock solution. The solution is more concentrated than the working stock. If tasked with making 128 mL of a 1x solution of PBS, how many mL of the stock solution would be needed? Report your answer in standard decimal notation rounded to one decimal place.arrow_forwardCHCASE Dashboard Item 3 A student modek four types of land use in a city. Each model has the same amounts of soil, pebbles, and gravel. A bottle is placed under each model to represent an aquifer, as showWn. Item 4 Item 5 Item 6 Item 7 Aqulfer Aquifer Item 8 Model P Model Q Item 9 Item 1 Item Item Aquifer Aquifer Model R Model S Which model represents land use that will recharge groundwater in a city the most, and why? O Model P; it has green spaces with grass and plants. O Model Q; it has neighborhoods with large front yards. O Model R; it has buildings near paved roads and parking lots. O Model S; it has houses near large trees with deep roots.arrow_forwardA student prepares a 0.54 mM aqueous solution of crotonic acid (C,H,CO,H). Calculate the fraction of crotonic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. I % x10arrow_forward
- Lab Data Reevaluate your calculations. Collected Volume sodium carbonate (mL) 99.0 Molarity sodium carbonate (M) 0.10 Volume calcium chloride (mL) 100.0 Molarity calcium chloride (M) 0.10 Observations The substance turns from clear liquid to a cloudy white substance. Mass filter paper (g) 0.27 Mass filter paper + precipitate (g) 1.14 Calculated Observed mass calcium 0.87 carbonate (g) MY NOTES Identify limiting reactant Sodium carbonate Expected mass calcium carbonate (g) Percent yield (%) How to calculate theoretical yield and percent yield e Type here to search 近arrow_forwardA student prepares a 0.28 mM aqueous solution of acetic acid (CH,CO,H). Calculate the fraction of acetic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. |% x10arrow_forwardA small business brings in a revenue of $60000 for the week. The payroll for the week is $20000. The owner spends $1000 on the company anniversary party. Of what is left, one third is donated to a local charity, and $500 less than half the amount that went to the charity goes to building repairs. What is the final amount of profit for the week? Question 5 options: $20000 $7000 $13000arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY