
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
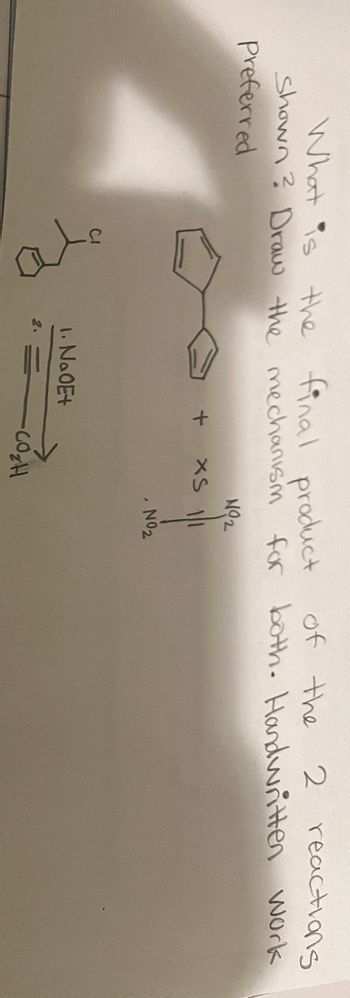
Transcribed Image Text:What is the final product of the
2 reactions
Shown? Draw the mechanism for both. Handwritten work
Preferred
ото
+
1. Na0E+
2.
NO2
XS
NO2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- From the following reaction are step 1 and 2 correct?, explain why or why not and draw the whole mechanismarrow_forwardWhat is(are) the major final product(s) formed during the following sequence of reactions? 01 11 V 1.0₂ 2. (CH₂)₂S C₂H₂O 2 identical molecules ||| =PPhy < IV орнаarrow_forwardWhich of the given reactions would form meso product? H₂O, H2SO4 III m CH3 CH₂ONa CH3OH || H₂O, H2SO4 CH3 1. LiAlH4, THF 2. H₂O CH3 IVarrow_forward
- 3. The following three SNAR transformations follow different mechanisms. Draw an intermediate or transition state for each reaction that relates the correct mechanism. Write a short sentence as to why that is the correct intermediate for the reaction. Reaction 1: Reaction 2: NO2 NO2 NO₂ + NaOMe NO2 + NaOH2 1. aq NaHCO3. 100 C 2. H₂O+ OMe NO2 NO₂ OH ŇO₂ NO2 Reaction 3: CFa Br CF3 8--2 + NaNH2 NH3 NH₂ Intermediate/transition state Intermediate/transition state Intermediate/transition statearrow_forwardDetermine the mechanism of nucleophilic substitution of each reaction and draw the products, including stereochemistr CH₂CH3 CI Br + CN d. + CH3COOH a. acetone + -OCH3 e. DMF f. b. C. ||||| a ||||| Br H Br CH2CH2CH3 + CH3OH DMSO H CH3 CI Br + OCH₂CH3 + CH3CH₂OHarrow_forwardExamine each reaction. Determine the mechanism (E1, E2, SN1, or SN2) for each reaction. SN1 E2 E1 SN2 CH2CH3 OocCHa)s CH2CH3 CH2CH3 А. Br major product Br DBU В. OOCH,CH, C. „Br Ooc(CH)a D.arrow_forward
- 9. Complete Reactions. Provide only the major product for E2&SN2 reactions. E1/SN1 provide all possible products. NaOH Br provide mechanism CI Provide mechanism + CI NaOCH 3 KCNarrow_forwardWhat is the product of the illustrated mechanism? A B C Darrow_forwardc. Snl reactions dominate under polar protic solvents. Increasing the polarity of the solvent will increase the rate of the reaction because the polar solvent will stabilize the dispersed charges on the transition state more than it will stabilize the neutral reactant. Label areas of ô* and & on the molecules of the transition state of the SN1 reaction below: Me Me Ме Me Me H20 Br -Br OH + НО \'H Et Et RDS Et H Et Et transition state not isolable carbocation intermediate not isolablearrow_forward
- 1. What is final product? 2. What are the 4 reactions & steps in mechanism (show product and mechanism steps)? 3. It is kinetic or thermodynamic?arrow_forward7. Which of (a)-(d) is least likely as an intermediate or product in the following reaction? CH cat. H* + HONH2 А. В. С. D. HO NHOH Ht OH N. но ONH2 HO N `H `H. H.arrow_forwardSECTION 6: Show the mechanism of each reactions with arrows and intermediates form as in a. b. C. HBr Br₂ Cl₂, H₂O Br Br CI Br OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning