
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Is it possible for alkynes to exhibit cis/trans isomerism? Explain your answer.
Expert Solution
arrow_forward
Step 1
Cis-Trans isomerism is possible only in the case of alkenes.
Alkynes can't exhibit cis/trans isomerism.
arrow_forward
Step 2
Cis isomer occurs when the same groups are present adjacent to each other on both sides of the double bond.
And trans-isomer occurs when the same groups are present opposite to each other on both sides of the double bond.
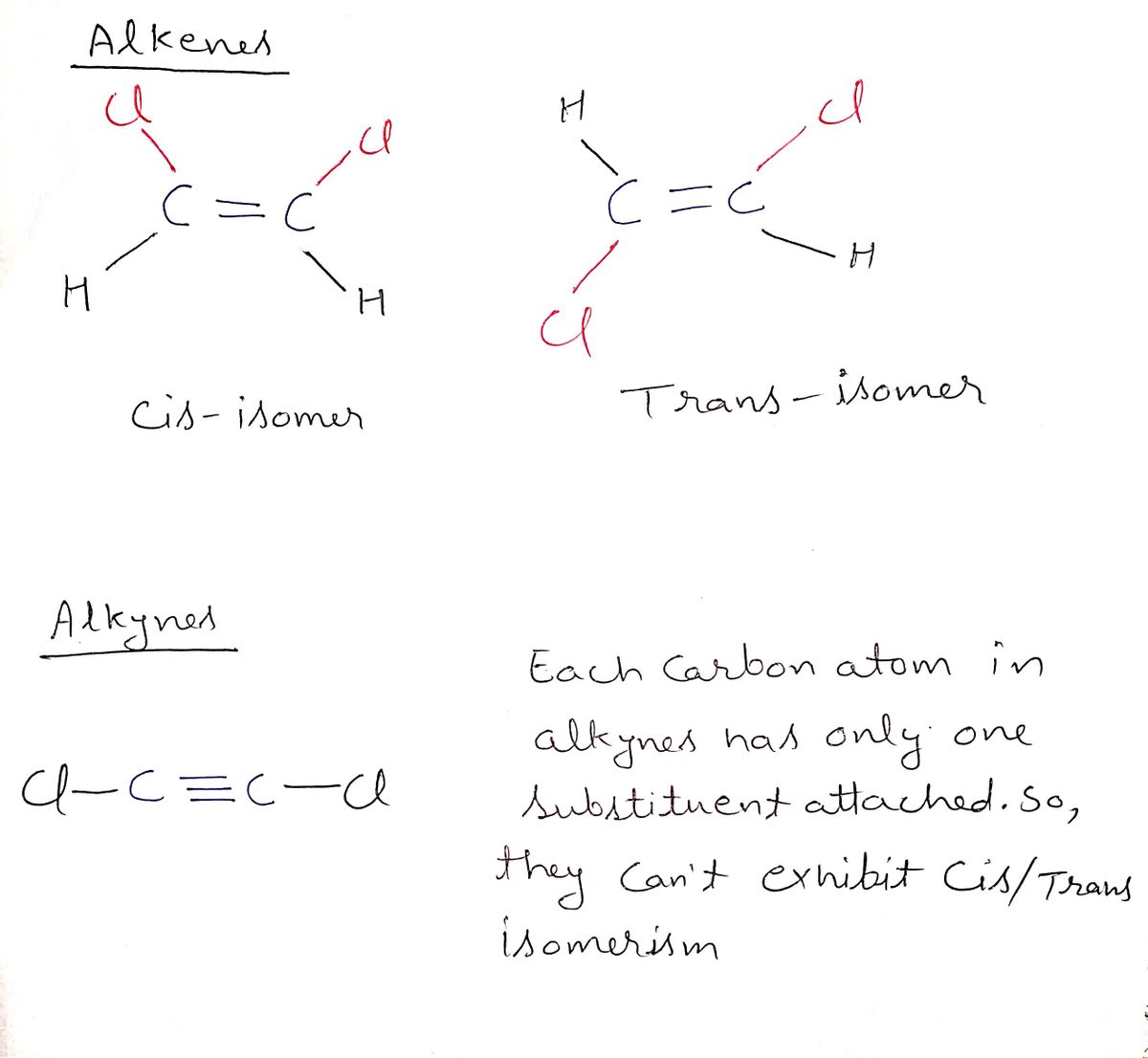
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An alcohol has the formula C4H9OH. How many isomers can exist for this alcohol?arrow_forward7. Draw the structures for the following hydrocarbons. Hydrocarbon Structure Hydrocarbon Structure Cyclohexane (C6H12) Benzene Cyclohexene Toluene (C7H8)arrow_forwardFor every one double bond in a structure of a hydrocarbon, there is one less hydrogen than found in an alkane with same number of carbons.arrow_forward
- Explain how the boiling point of substances varies in a homologous series of alkanes? Provide examples.arrow_forwardWrite Lewis structures and name the five structural isomers of hexane.arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Name 6 Structural isomers of C5H10. They should three alkene and two cycloalkanearrow_forward
- (5c-201-5) Draw a skeleton structure and give the IUPAC name of a molecule with one alcohol group, 5 total carbons, and one methyl substituent group.arrow_forwardWhich of the following intermolecular forces is responsible for the boiling-point trends in alkanes?arrow_forwardDraw skeletal structures for the cyclopropane (three- membered ring) isomers with a formula of C5H10. Note: cyclopropane is a carbon-carbon ring with three carbons.arrow_forward
- 12. Identify (adraw a circle and label the class) the classes of organic molecules represented in the following molecule Ноarrow_forwardWhat happened to the sigma bonds when a cycloalkane forms?arrow_forwardUse the following information to answer the question A student added bromine solution to a hydrocarbon sample that contained an isomer of C6H12 (E) in the absence of light. After shaking the sample, the student noticed that the colour of the bromine solution changed from orange to colourless. 6. An interpretation that could be made from the student's observations is that the hydrocarbon sample is and the IUPAC name of the sample could be ii. I The statement above is completed by the information in row: Row i ii A saturated B saturated hex-2-ene cyclopentane C unsaturated hex-2-ene D unsaturated cyclopentane -12 markelarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY