
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
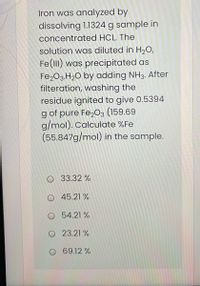
Transcribed Image Text:Iron was analyzed by
dissolving 1.1324 g sample in
concentrated HCL. The
solution was diluted in H2O,
Fe(lII) was precipitated as
Fe203.H2O by adding NH3. After
filteration, washing the
residue ignited to give 0.5394
g of pure Fe,03 (159.69
g/mol). Calculate %Fe
(55.847g/mol) in the sample.
O 33.32 %
O 45.21 %
O 54.21 %
O 23.21 %
O 69.12 %
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 0.753-g sample of a chlorocarbon compound was analyzed by burning it in oxygen and collecting the evolved gases in a solution of NaOH. After neutralizing, the sample was treated with 25.74 mL of a 0.264 M AgNO3 solution. This precipitated the chloride (Cl-) out as AgCl and left an excess of AgNO3. The excess AgNO3 was titrated with 0.1 M KSCN and required 16.47 mL to reach the endpoint in a Volhard titration. Calculate the % w/w Cl– (35.45 g/mol) in the sample. Provide your answer to 2 places after the decimal point and without units. Reactions: Cl– + Ag+ → AgCl(s) Reaction 1 Ag+ + SCN– → AgSCN(s) Reaction 2 HINT: This is an example of a back-titration. Steps to success: First, calculate the TOTAL amount (in moles) of Ag+ added from the volume and molarity of the silver nitrate solution. Second, determine the EXCESS amount of Ag+ from the reaction with thiocyanate (Reaction 2 above). Third, calculate the…arrow_forwardA solution of oxalic acid dihydrate (H₂C₂O₄・2H₂O) with a known concentration of 0.400 M H₂C₂O₄・2H₂O is titrated with a 0.333 M NaO solution. How many L NaOH are required to reach the second equivalence point with a starting volume of 65.0 mL H₂C₂O₄・2H₂O, according to the following balanced chemical equation: H₂C₂O₄・2H₂O + 2NaOH → Na₂C₂O₄ + 4H₂Oarrow_forwardAnswer the following.arrow_forward
- The zinc content of a 1.03 g ore sample was determined by dissolving the ore in HCl, which reacts with the zinc. The excess HCI is then neutralized with with NaOH. The reaction of HCl with Zn is shown. Zn(s) + 2 HCl(aq) ->> ZnCl₂ (aq) + H₂(g) The ore was dissolved in 150 mL of 0.600 M HCl, and the resulting solution was diluted to a total volume of 300 mL. A 20.0 mL aliquot of the final solution required 8.94 mL of 0.530 M NaOH to neutralize the excess HCl. What is the mass percentage (%w/w) of Zn in the ore sample? %w/w = % Znarrow_forwardA quantitative analysis for ethanol, C2H60, is accomplished by a redox back titration. Ethanol is oxidized to acetic acid, C2H402, using excess dichromate, 3+ Cr207 2-, which is reduced to Cr3*. The excess dichromate is titrated with Fe2*, giving C and Fe+ as products. In a typical analysis, a 5.00-mL sample of a brandy is diluted to 500 mL in a volumetric flask. A 10.00-mL sample is taken and the ethanol is removed by distillation and collected in 50.00 mL of an 2- acidified solution of 0.0200 M K2Cr207. Titration of the unreacted Cr207 requires 21.48 mL of 0.1014 M Fe2*. Calculate the %w/v ethanol in the brandy. Which is the titrant in this problem? C2H402 Fe2+ C2H60 O Cr207 2-arrow_forwardCalculate the% of Ce (MA 140.115 g / mol) that contains a sample with a mass of 4.37 g that was dissolved and treated with an excess of iodate to precipitate it as Ce (I03) 4. The precipitate was washed, dried and calicoed, yielding 0.104 g of CeO2 (MM 172.114 g / mol). What is the% by weight of Ce in the sample?arrow_forward
- 1. A solution of HCIO, was standardized by dissolving 0.3745 g of primary standard grade HgO in the solution of KBr. The liberated OH required 37.79 mL of the acid to be neutralized. Calculate the molarity of HCIO.. HgOs) + 4Br + H,0 > HgBr. + 20Harrow_forwardExcess solid Ni(OH)2 was added to water and the resulting solution was allowed to stand for several days. The solution was then filtered to remove excess solid Ni(OH)2. A 0.250 L aliquot of the solution was then titrated with 10.54 mL of a standardized 0.000500 M HCl solution. What is the Ksp of Ni(OH)2?arrow_forwardFe3+ in 50 mL solution, which is known to contain Fe3+, was precipitated as Fe(OH)3 and after necessary procedures, it was brought to constant weight as Fe2O3. Since the weight is found to be 0.3994 g, find the Fe3+ concentration in the solution in terms of molarity. (Fe: 55.85, O: 16.00 g / mol)arrow_forward
- The protein in a 0.2976 -g sample of cheese is determined by the Kjeldahl method. The sample is digested with H2SO4, the resulting solution made basic with NaOH, and the ammonia distilled into 50.00 mL of 0.09756 M HCl. The excess HCl is then back titrated using 39.87 mL of 0.05345 M NaOH. Calculate the % N and % protein of the sample.arrow_forwardMark and John, two vinegar enthusiasts, are each tasked to determine the acetic acid content of their respective vinegar concoctions by titration. First, a 1 M-labeled KOH solution was standardized against the KHP (MW = 204.22 g/mol) standard that is 99.4% pure. In the process, 0.540 g KHP was found to require 2.80 mL of the KOH solution to completely react up to the phenolphthalein endpoint. Then, Mark and John both prepared their samples by taking 10.0-mL aliquots of each vinegar and diluting them to 25.0 mL. Using the same titrant and indicator, Mark’s vinegar required 18.60 mL of the standardized titrant to reach the endpoint, while John’s vinegar required 16.50 mL of the same titrant to reach the same endpoint. Question: What is the acetic acid concentration of Mark’s and John’s vinegar in molarity given that the Concentration of KOH is 9386 M?arrow_forwardA sample contains MgCl2 (95.211 g/mol) and KCl (74.551 g/mol). A 6.6933-g sample was dissolved in enough H2O to make 250.0-mL solution. A 50.00-mL aliquot was treated with AgNO3 to precipitate the chlorides and after proper collection and drying gave a precipitate weighing 1.9620-g. Another 50.00-mL aliquot was tested for its Mg2+ content by precipitating it with NH4+ and PO43- giving an Mg2P2O7 (222.551 g/mol) precipitate weighing 0.5186 g with FW AgCl 143.32 g/mol. What is percent KCl in the sample?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY