
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The proton nmr is
1H Septet between 3.5 and 4
3H singlet at about 3.4
6H doublet at about 1.2
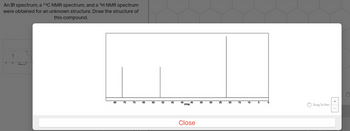
Transcribed Image Text:An IR spectrum, a ¹³C NMR spectrum, and a 1¹H NMR spectrum
were obtained for an unknown structure. Draw the structure of
this compound.
PPM
Close
Drag To Pan
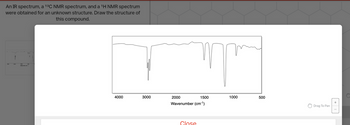
Transcribed Image Text:An IR spectrum, a ¹³C NMR spectrum, and a ¹H NMR spectrum
were obtained for an unknown structure. Draw the structure of
this compound.
Handal
4000
3000
2000
T
Close
1500
Wavenumber (cm-1)
1000
500
Drag To Pan
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me identify the structure of this NMR! Thank you!! 11 10 8. 7 6 5 4 3 2 1 HSP-00-768 ppmarrow_forwardThe MS and 13C NMR spectra for two constitutional isomers with 4 degrees of unsaturation are shown below. Determine the molecular structures of the two constitutional isomers.arrow_forwardTHIS CAN NOT BE HAND DRAWN! MUST USE A COMPUTER or DIGITALLY ILLUSTRATEarrow_forward
- Explain why 2-chloropropene shows signals for three kinds of protons in its 1H NMRspectrum? Draw the structure and predict the approximate values for 1H-NMR spectra.arrow_forward12 olot Sketch of ¹H NMR spectrum: ¹H NMR ofot 220 13C NMR sketch Chemical Shift (ppm) Chemical Shift (ppm) 0 0arrow_forwardWhich of the following compound is consistent with the following 13CNMR spectrum? To preview the image click here 80 до A ОН 70 60 ОН B 50 40 PPM 30 Хон 20 10 ОН D оarrow_forward
- Based upon the following NMR SPectrum please list the chemical shifts, multiplicity, and assignments of the major peaksarrow_forwardDetermine the structures of these spectrums (each has an unsaturation of 5)arrow_forwardDraw the NMR spectra you would expect for the following compounds. Note that you must put the peaks at the proper range of chemical shift, with proper integral labeled below the peak, and proper spin-spin splittingarrow_forward
- Identify the significant absorption peaks by labeling them right on the spectrumand includethe spectrum in your laboratory report. Absorption peaks corresponding to the followinggroups should be identified: C—H (SP3) C—H (SP2) C—H (aldehyde) O—H C=O C=C (aromatic)aromatic substitution pattern C—OC—X (if applicable)arrow_forwardLabel 3 peaks and what functional groups and their wavelength they could possibly correlate with in.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY