
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
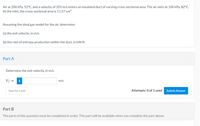
Transcribed Image Text:Air at 200 kPa, 52°C, and a velocity of 355 m/s enters an insulated duct of varying cross-sectional area. The air exits at 100 kPa, 82°C.
At the inlet, the cross-sectional area is 11.57 cm².
Assuming the ideal gas model for the air, determine:
(a) the exit velocity, in m/s.
(b) the rate of entropy production within the duct, in kW/K.
Part A
Determine the exit velocity, in m/s.
V2 =
i
m/s
Save for Later
Attempts: 0 of 1 used
Submit Answer
Part B
The parts of this question must be completed in order. This part will be available when you complete the part above.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Air flows Skeadily through a themally insulated duct of Varyıng Cress- Sectonal area . The air enters at 52°C , 200 kla, and 355 m/Sec, and exits at 77°C and kla. The inlet oross- Sechonal area is 6.57 cm?. Using the ideal gas model, but without assuming constant Specific heats, Calculate the rate of entropy production within the duct in kW/°K using 5 signifigant Agures.arrow_forward4) Hot combustion gases enter the nozzle of a turbojet engine at 350 kPa, 1007 °C and 95 m/s, and they exit at a pressure 100 kPa. Assuming an isentropic efficiency of 95 percent and treating the combustion gases as air determind a- The exit velocity b- The exit temperaturearrow_forwardTwo insulated Tanks are connected with an insulated pipe and a valve. The valve is open and steam flows from Tank A to tank B in a quasi static proccess until the valve is closed. The specific entropy in each tank is maintained the same, but the total entropy in the system is changed , explain why .arrow_forward
- A- Air at 15°C and 1bar occupies 0.02 m³. The air is heated at constant volume until the pressure is 5bar, and then cooled at constant pressure back to the original temperature. Calculate the net heat flow to or from the air and the net entropy change. Sketch the process on a T-s diagram. (8.5marks)arrow_forwardAir is compressed in an adiabatic compressor. Inlet and outlet conditions are 120kPa, 30 oC and 20 m/s; 1.4 MPa, 530 oC and 80 m/s. The surrounding air temperature is 25 oC. The inlet cross-sectional area is 0.013 m2. Assume Cp = 1005 J/kgK, R = 287 J/kgK, k = 1.4. Enter the amount of heat loss due to irreversibility resulting from the heat transfer from the compressor in kW (correct up to one decimal place.)arrow_forwardA refrigerator transfers 1kJ of heat from a cold region at -20°c to hot region at 30°c. If COP of refrigerator is 4, total entropy change of region's will be:- A. 1.72 X 10-4 KJ/K B. 0.173 KJ/K Ос. 1.7 X 102 кЈ/К D. 0.9263 KJ/Karrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY