
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Transcribed Image Text:|||
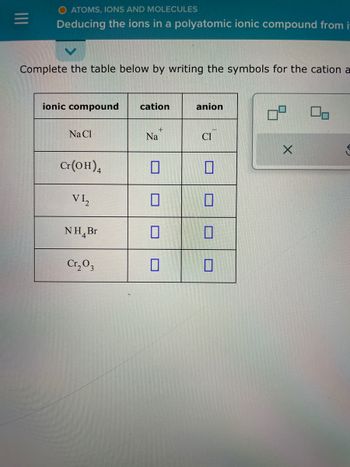
O ATOMS, IONS AND MOLECULES
Deducing the ions in a polyatomic ionic compound from i
Complete the table below by writing the symbols for the cation a
ionic compound
Na Cl
Cr(OH)4
VI₂
NH Br
Cr₂ 03
cation
+
Na
П
0
0
anion
Cl
0
0
0
90
Expert Solution
arrow_forward
Step 1: Defining Ionic compounds
Answer:
Compounds in which oppositely charged ions combined together via strong electrostatic attraction force are called as ionic compounds.
Step by stepSolved in 3 steps with 13 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name of compoundarrow_forward(a) Flake white was a white pigment used by Renaissance artists, which was composed of a combination of lead(II) carbonate and lead(II) hydroxide. What are the correct chemical formulas for these two compounds? PbHCO3 Pb(OH)2 Pb2CO3 PbO Pb(OH)4 PbCO3arrow_forwardFill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation Cu 2+ Mg 4+ Pb 2+ Ca anion 2- SO. 3 NO₂ IO Bro 4 some ionic compounds empirical formula 11 0 name of compound 0 0 X 00 Śarrow_forward
- If 2.00 grams of Epsom salts are allowed to dehydrate in a warm oven overnight, the mass of the remaining powder is 0.98 grams. If you assume that the powder is MgSO4 only, what is the formula for this batch of Epsom salts? atomic weights: O – 16.00 amu; Mg – 24.31 amu; S – 32.07 amuarrow_forwardOnly typed solutionarrow_forwardCalculate the number of formula units present in 1.34 g of Sr(NO2)2.arrow_forward
- Explaining to me in great detail how to name Sr(OH)2 and Fe(OH)3 compounds. What type of compounds are they? What are the charges on the metals? How are the nomenclature rules different for these two compounds?arrow_forwardThe recommended daily allowance of the niacin (vitamin B3) is 16 mg per day for man. The molecular formula for niacin is C6H5NO2. Calculate the number of molecules of niacin in 16 mg.arrow_forwardWhat is the mass of 5.34 x 10^23 molecules of sodium hydrogen carbonate. So far I have Na:22.99 + H1.00 + C12.01 + O3 15.99(3) =47.97 NaHCO3 83.97g/molarrow_forward
- ||| O ATOMS, IONS AND MOLECULES Naming ionic compounds with common polyatomic ions Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation 2+ Mg 3+ Cr 4+ Pb anion 3- PO OH so Some ionic compounds empirical formula 0 name of compound 0 0 X Do 3arrow_forwardFill in the name and empirical formula of each ionic compound that could be formed from the ions in this table:arrow_forwardDetermine mass percent of sodium in Na2PO4 Na: 22.990 amu P: 30.974 amu O: 15.999 amu 0.36 36.5% 0.45 32.6%arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY