
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Interpret the 1H NMR spectrum of Indole-3-Carboxaldehyde
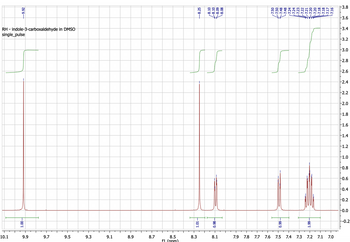
Transcribed Image Text:-9.92
RH - indole-3-carboxaldehyde in DMSO
single_pulse
10.1
1.00-
9.9
9.7
9.5
9.3
9.1
8.9
8.7
8.5
f1 (nnm)
8.3
-8.25
-8.10
-8.10
-8.09
-8.08
0.98-
8.1
on
-7.50
-7.50
7.48
-7.48
7.24
-7.24
7.23
-66'0
7.9 7.8 7.7 7.6 7.5
77%
7.21
-7.20
7.20
7.18
-7.18
-7.17
7.16
7.4 7.3 7.2 7.1 7.0
+3.8
-3.6
-3.4
-3.2
-3.0
-2.8
-2.6
-2.4
-2.2
-2.0
-1.8
-1.6
-1.4
+1.2
+1.0
-0.8
-0.6
-0.4
-0.2
-0.0
-0.2
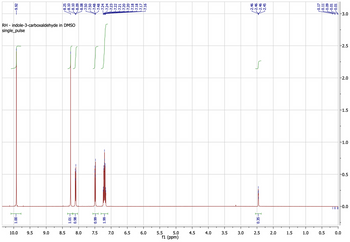
Transcribed Image Text:-9.92
RH - indole-3-carboxaldehyde in DMSO
single_pulse
FOOT
10.0 9.5
-8.25
8.10
-8.10
-8.09
8.08
-7.50
-7.50
9.0
8.5
TT
1.01
+86*0
8.0
7.48
7.48
H
-7.24
-7.24
-7.23
-7.22
-7.21
-7.20
-7.20
660
66 T
7.5
2.4
7.0
6.5
-7.18
-7.18
-7.17
7.16
6.0
5.0
f1 (ppm)
5.5
4.5
4.0
3.5
3.0
2.46
-2.46
-2.46
-2.45
0.35
2.5
2.0
1.5
1.0
0.17
-0.11
-0.09
-0.01
-0.01
0.5
-3.0
-2.5
-2.0
-1.5
F1.0
-0.5
TI 0.0
0.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The 1H-NMR spectrum of Compound C shows five signals – δ 2.38 (1H, dt), 2.72 (1H, dt), 5.34 (1H, t), 5.49 (2H, ddd), 6.27 (2H, dd) ppm. Its 13C-NMR spectrum has four signals – δ 26, 58, 127, 129 ppm. In the compound’s mass spectrum, the M+1 peak appears at m/z = 115. An M+2 peak, whose intensity is roughly one-third that of the M+1 peak, also appears. Suggest a structure for this compound.arrow_forwardShown below is the H-NMR spectra of acetaminophen. Draw the structure and correlate each hydrogen in the molecule with the various peaks in the spectra.arrow_forwardIn detail, explain how to separate a mixture of naphthalene, byphenyl-4-carboxylic acid, and 4-chloroaniline.arrow_forward
- Using the 1H NMR complete the following table and draw the structure.arrow_forwardcompare and construct the nucleophilic reactions of hcl with propanone, propanal and propan-1-0arrow_forwardwrite structural formulas for the products that form when propanal reacts with phenylhydrazine. draw a step by step mecharrow_forward
- Which of the following functional groups are present in 2-hydroxy-1,2-diphenylethan1-one? Group of answer choices aldehyde mono-substituted phenyl alkene carboxylic acid nitro ketone amine ortho-disubstituted phenyl ether alkyl ester amide hydroxylarrow_forwardThe product of reaction between formaldehyde with 2-methyl propanal give 2,2-dimethyl-3-hydroxy propanal 2,2-dimethyl-3-hydroxy propanone 2,2-dimethyl-3-hydroxy propanoic acid 2,2-dimethyl-3-hydroxy propanol 4-phenyl-3-buten-2-one prepared from reaction between Benzaldehyde with acetic acid Benzaldehyde with acetaldehyde Benzaldehyde with formaldehyde Benzaldehyde with acetone Which predict product for reaction between heptane-6-one-1-al with sodium hydroxide * 1-(2-hydroxycyclopentyl) pentanone 1-(2-hydroxycyclopentyl) butanone 1-(2-hydroxycyclopentyl) ethanone 1-(2-hydroxycyclopentyl) propanonearrow_forwardAs a method for the synthesis of cinnamaldehyde (3-phenyl-2-propenal), a chemist treated 3-phenyl-2-propen-1-ol with K2Cr2O7 in sulfuric acid. The product obtained from the reaction gave a signal at δ5 in its 13C NMR spectrum. Alternatively, when the chemist treated 3-phenyl-2-propen-1-ol with PCC in CH2Cl2, the 13C NMR spectrum of the product displayed a signal at δ193.8. (All other signals in the spectra of both compounds appeared at similar chemical shifts.) (a) Which reaction produced cinnamaldehyde? (b) What was the other product?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY