
EBK A SMALL SCALE APPROACH TO ORGANIC L
4th Edition
ISBN: 9781305446021
Author: Lampman
Publisher: CENGAGE LEARNING - CONSIGNMENT
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
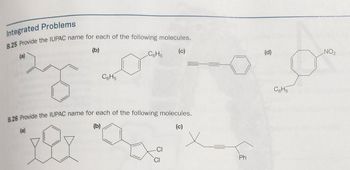
Transcribed Image Text:Integrated Problems
B.25 Provide the IUPAC name for each of the following molecules.
(b)
C6H5
(b)
C6H5
B.26 Provide the IUPAC name for each of the following molecules.
-CI
(c)
CI
(c)
Ph
(d)
C6H5
NO₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the formula for the following. (a)Conjugate base of H2O (b)Conjugate base of H2PO4– (c)Conjugate acid of HS –arrow_forwardCH3NH2 is a (a)Arrhenius acid. (b)Arrhenius base. (c)Bronsted-Lowry acid. (c)Bronsted-Lowry base. (e)Louis base.arrow_forward(a) Ionic Solids do not dissolve in non-polar solvents. (TRUE OR FALSE) (b) H3AsO4 is a weak acid. (TRUE OR FALSE) (c) Once a chemical reaction has come to equilibrium, the reaction comes to a complete halt.(TRUE OR FALSE)arrow_forward
- In the following reaction in aqueous solution, the base reactant is and its conjugate acid product is CH3COOH(aq) + NH3(aq) CH3COO (ag) + NH4 (ag) O NH3: NH4 NH3: CH3COO CH3COOH; NH4 CH3COOH; H30+ CH3COOH; CH3CO*arrow_forward(a) Predict the products of the following acid-base reactions using curved-arrow mechanisms to indicate electron flow. (b) Indicate the acid, base, conjugate acid, and conjugate base of each reaction. (c) Indicate whether the reactants or products are favored at equilibrium a) CH,COOH CH3O b) CH,CH,OH H2Narrow_forward(a) Wr ite an equat ion for the react ion in whichH2C6H7O5-(aq) acts as a base in H2O(l). (b) Write an equationfor the reaction in which H2C6H7O5-(aq) acts as an acidin H2O(l). (c) What is the conjugate acid of H2C6H7O5-(aq)?What is its conjugate base?arrow_forward
- In water solution, acetylsalicylic acid acts as a weak acid:CH3COOC6H4COOH (aq) + H2O (l) ⇌ H3O+ (aq) + CH3COOC6H4CO2- (aq) Are the following Brønsted-Lowry acids or Brønsted-Lowry base H2O (l) CH3COOC6H4COOH (aq) H3O+ (aq) CH3COOC6H4CO2- (aq)arrow_forwardGive detailed Solution with explanation needed..please explainarrow_forward(c) NH2 Draw Your Solution HA (cat.)arrow_forward
- 1) Complete the following reactions and identify the acid, base, conjugate acid and conjugate base. CH;CH,NH2 (aq) + H2O (1) a) CH;COOH (aq) + H,O (I) b) c) Identify the conjugate base and conjugate acid of each of the following. Conjugate Base Molecule Conjugate Acid HCO3 H20 HSO4- H2PO4arrow_forwardWrite equations for the following acid-base reactions.(a) HCOOH + -CN (b) CH3COO- + CH3OH(c) (CH3)2CHOH + NaNH2 (d) NaOCH3 + HCNarrow_forwardFor the following acid-base reaction, (1) predict the products, showing both reactants and products complete Lewis structures and arrows showing electron flow; (2) label each structure with the lowing: Bronsted acid, Bronsted base, conjugate acid, conjugate base; (3) give a brief definition of a ronsted acid and Bronsted base; (4) predict the direction of the equilibrium and justify your answer. HC0OH + CH3 Nta PRん106Y pkb = 3.36arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT