
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
[V1&2] Instructions: Consider the following energy diagram for the conversion of A to G. (refer to the photo below)
- Which points on the graph correspond to transition states?
- Which points on the graph correspond to reactive intermediates?
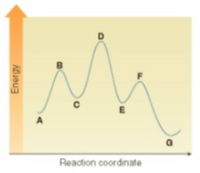
Transcribed Image Text:A
Reaction coordinate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The substitution of an Iby a Clon H3C-I can occur by two possiblemechanisms:Mechanism I: step 1. H3CI → H3C+ + I- slow stepstep 2. H3C+ + Cl- → H3CCl fast stepMechanism II: step 1. H3CI + Cl- → H3CClI slow stepstep 2. H3CClI → H3CCl + I- fast stepa.) Write a rate law for each reaction.arrow_forwardWhat is the intermediate of the reaction based on the image below?arrow_forward← Chapter 8 Question 9 of 17 View Policies Show Attempt History Current Attempt in Progress X Incorrect. H₂C Draw the major product(s) for the following reaction. If a racemic mixture of enantiomers is expected, draw both enantiomers (you can use copy/paste to save drawing time). ? Hint CH₂ H3O* Edit Drawing ave for Later eTextbook and Media -OH 0/1 ||| : Assistance Usedarrow_forward
- Which of the following correctly describes a key step of the reaction shown in the box? 1. ELONA 2 2. HаО, НСІ OEt :o: :o: :o: A) Et H3C p: 1 :o: :o: :o: :o: B) H3C Et H3C :o: :o: :0: + C) H2C H3C :o: H :0: :0: :o: D) H3COEt H3C OEt O A O B OD O=arrow_forward5. Which of the following statements regarding the reactions A and B (shown below) is the best? 0 heat 0 heat B A . Both reaction A and B are feasible because frontier orbitals of reactants can overlap in-phase, allowing the simultaneous formation of two o bonds. . Both reaction A and B are NOT feasible because frontier orbitals of reactants can NOT overlap in-phase. Only reaction A is feasible because frontier orbitals of reactants can overlap in- phase, allowing the simultaneous formation of two o bonds. Only reaction B is feasible because frontier orbitals of reactants can overlap in- phase, allowing the simultaneous formation of two o bonds.arrow_forward7.arrow_forward
- 2. a) Draw mechanism arrows to show how the reactants are converted to the products. b) Using the pKa values in Table 6.1, determine which side of the equilibrium will be favored. H-CI CląC Cl3C HO, H EH H Earrow_forwardAt what temperature does C₂H₂Cl₂ have a density of 0.812 g/L at 1.17 atm? Please include calculations so I can understand how you got the answerarrow_forwardI need help with question 1 and 2arrow_forward
- Mechanism: A reaction mechanism for the following reaction is shown below. H+ CEN CEN: N-H || -C-OH H₂O, H* Step 1 Step 3 N-H C-OH C=N-H H₂O Step2 .C=N-H H-O-H Semse anls a) The overall reaction is an example of b) Step 1 is c) Step 3 is d) Draw in the curved arrows for each step. e) Identify the nucleophiles and electrophiles where appropriate. f) Fill in the reaction energy diagram. C=N-H H₂O: rate determining step E个 reaction progress →arrow_forward6. Consider the following acid /base reaction. N-Me Me CN-H - + Me. Me - N | me Me a) Draw the two products of this reaction b) will the reactants or products be favored at equilibrium? Briefly justify your answer by referencing specific sructural features of the two bases in the above reactionarrow_forward\/C)0.Bm HB 4 1. What reagent can be used to form the product in each of the following? но | || H-C-C-H Н WA Н- нон -C-C-H Н Н нно -С-С-С-О I Н Н — - - - HO H-C-C-OH H H OH H | | | H-c-c-c-H | | | Hнн HHO H-C-C-C-H Н Н What is the product of this reaction? H OH H-C-C-CH3 H2CRO4 H CH3 What is the name of the bond formed when methanethiol is oxidized? CHz-S-H + H-S-CH3 — CH-S-S-CH3 + H2O Identify the acetal and hemiacetal functional group in the following OF CH охо OH OPE Guitkwot И www CH₂ OH H ко Calculate the molecular formula for each of the following. 985 НОarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY