
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
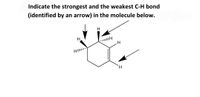
Transcribed Image Text:Indicate the strongest and the weakest C-H bond
(identified by an arrow) in the molecule below.
H
H.
.....
HI.
TH.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You will not find “hydroxide” in the stockroom, but you will find sodium hydroxide (NaOH) andpotassium hydroxide (KOH). Lithium hydroxide (LiOH) is expensive and used in spacecraft airfilters since hydroxide reacts with carbon dioxide, and lithium is lighter than sodium or potassium.Cesium and francium hydroxides are very expensive and little used. Is this information consistentwith your answer to the previous question?arrow_forwardFor each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. name dimethyl ether iodomethane acetic acid compound formula or Lewis structure H T H-C-Ö-C-H I H HILIA CH₂I | H H :O: | || H-C-C-O-H Between molecules of the compound? O yes O no O yes O no hydrogen-bonding force O yes O no Between molecules of the compound and molecules of water? O yes Ono O yes O no O yes O no X Sarrow_forwardthway | Algebra.. Pt Periodic Table - Pta... Scientific Calculator Gravitational Force... Conservation of Mo... Part 1-Combustion of Methane Equation for Enthalpy AH = Hbonds broken- Haonds formed The following table is a list of selected bond energies. The bonds present in this table are common bonds for combustion reactions Selected Bond Energies Bond Bond Energy Bond Bond Energy (kl/mole) (kl/mole) H-H 436 C=0 799 0-0 495 C-C 348 O-H 463 C=C 614 C-H 413 C-C (aromatic) 519 C-O 358 N=0 623 Use the following balanced equation and chart to complete the bond energy calculatons for the fuel methane. CH+202-CO2+2 H20 Compound Total Bond Energy Oxygen, O, 495 kJ/mol Carbon Dioxide, CO, 1598 kJ/mol Water, H,O 926 kJ/molarrow_forward
- Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. chemical symbol, chemical formula or Lewis structure substance boiling point H:0: H do II. I H-C- C-N- C- H (Choose one) e H H H H. H :0: H. .. B H-C- C-0 -C- H (Choose one) 8 H. H. F2 (Choose one)e :NEN- O: (Choose one) e Explanation Check . Terms of UseI Privacy C Center Accessibty O 2022 McGraw Hill LLC. All Rights Reserved DII F9 F10 08 F3 17 F5 F6 F4 esc F2 & %23 2$ delete @ 7 8. 3 4 1 Y Q W E tab H J K return G .. •-arrow_forwardH зн. + H:Y: H The Lewis representation above depicts a reaction between hydrogen (blue) and a main-group element from group | (red). In this representation, each Y atom needs bond(s) with atoms of H. ]electron(s) to complete its octet, and gains these electrons by forming There are| Junshared electron pair(s) and | bonding electron pair(s) in the product molecule. The bonds in the product are |arrow_forward4. Polarity: Circle the molecules that are polar and draw the delta notation around the polar bonds within the molecules that are polar. H3C CH3 H H H- H H. H H H- -H- H- H. H H H H. о N EN H- H. H.arrow_forward
- Number of Molecule valence electrons Formal Charge Electron-Group Geometry Molecular Geometry Resonance HSO4 S: S: Select one . S: Select one .. Select one XeTe4 Хе: Xe: Select one ... Xe: Select one .. Select one PHO P: P: Select one ... P: Select one .. Select one ... v PCIO P: P: Select one ... P: Select one .. Select one ..arrow_forwardFor each compound in the table below, decide whether there would be any hydrogen-bonding force between molecules of the compound, or between molecules of the compound and molecules of water. name formic acid compound dibromomethane acetic acid formula or Lewis structure :0: || H-C-O-H CH₂Br₂ H :0: | || H-C-C-O-H H Between molecules of the compound? 00 00 yes no yes no yes hydrogen-bonding force no Between molecules of the compound and molecules of water? 00 00 yes no yes no yes no Xarrow_forwardNumber of Molecule valence electrons Formal Charge Electron-Group Geometry Molecular Geometry Resonance BH3B" B: B: Select one ... B: Select one ... Select one . SbAt2Br Sb: Sb: Select one ... Sb: Select one .. Select one BCI4 B: B: Select one.. B: Select one .. Select one .. V NBr3F* N: N: Select one.. N: Select one ... Select one . AIHCI3 Al: Al:Select one ... Al: Select one . Select one .arrow_forward
- Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C H H - - chemical symbol, chemical formula or Lewis structure H :0: | || C 1 H | H H | || C-N- | H H - F₂ :NEN 0: :0: - H | C C-O- — H | C I H - H — - H - boiling point (Choose one) ✪ (Choose one) ✪ (Choose one) (Choose one) 09:39 6 00 Aarrow_forwardNumber of Molecular Geometry Resonance Molecule valence electrons Formal Charge Electron-Group Geometry Si: Select one .. Select one .. SIAT2CI2 Si: Si: Select one ... Pb: Select one ... Select one ... Pb: Pb: Select one . PbH3At Si: Select one ... Si: Select one .. Select one SiH¿Br2 Si: Si: Select one ... Si: Select one ... Select one . SİB12F2 Si: Select one ... Ge: Select one ... Ge: Select one ... GeBr3CI Ge: 00arrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance chemical symbol, chemical formula or Lewis structure B H:0: | || H-C-C-N- CH3 Br CaBr, H CH3 C H :0: H I IN H-C-C-C-H | | | D H H H CH₂- N― CH3 I CH3 boiling point (Choose one) v (Choose one) v (Choose one) (Choose one) ? Π ་ 00.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning