
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
14.
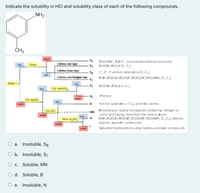
Transcribed Image Text:Indicate the solubility in HCl and solubility class of each of the following compounds.
NH2
ČH3
insol.
RCOO Na", R,NX' , some polyfunctional compunds
RCOOH, RSO,H (C,C;)
sol.
Ether
Litmus red (ag)
Litmus blue (aq)
1°, 2°, 3° amines (aliphatics) (C, C,)
ROH, RC(O)H, RC(O)R', RC(O)OR', RC(O)NH, (C, C)
sol.
Litmus unchanged (aq)
Water
sol.
A
RCOOH, RSO,H (>C;)
sol.
5% NaHCO,
Phenols
5% NaOH
insol.
sol.
insol.
B
Amines (aliphatics, > C), aromatic anines
Miscellaneus neutral compounds containing nitrogen or
sulfur and having more than five carbon atoms
ROH, RC(O)H, RC(O)R', RC(O)OR', RC(O)NH, (C C,), alkenes,
alkynes, aromatic compounds
5% HCI
insol.
MN
sol.
96% H,SO.
insol.
insol.
Saturated hydrocarbons, alkyl halides, aromatic compounds
O a. Insoluble, SB
O b. Insoluble, S1
О с. Soluble, MN
O d. Soluble, B
O e. Insoluble, N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is "?"arrow_forwardOptions for first blank: A. Exothermic B. Endothermic Options for second blank: A. Less B. More Options for third blank: A. Le chantelier’s principle B. Henry’s lawarrow_forwardEthanol and KCl both dissolve in water. what happens to the ionic bond when KCl dissolves? What types of interactions allow KCl to dissolve? What happens to covalent bonds in ethanol when it dissolves? What type of interactions allow ethanol to dissolve?arrow_forward
- 10:24 1 Done Introductory+Chemistry ill a 10. What mass of Li₂O is needed to react with 1,060 g of CO₂? Li₂O(aq) + CO₂(g) → Li₂CO3(aq),arrow_forward(10.10)Which of the following compounds has the longest carbon-carbon bond? C₂H₂ C₂H6 O C₂H4 O All of these have the C-C bonds with the same length.arrow_forward39arrow_forward
- A doctor has ordered for a week 0.35 grams of Carvedilol for a patient with high blood pressure. If your stock on hand consists of 25 mg tablets, how many tablets will you need for 1 day's treatment for the patient? QUESTION 13 O a. 2 O b. 14 O C. 7 O d. 20 O e. 1 QUESTION 14arrow_forwardFor a molecular species, its molar mass in g/mole would be the same number as its molecular mass in amu when: A. the temperature is increased by 100 oC B. there are an Avogadro’s number of molecules C. there are two dozen molecules D. the molecular species is dissolved in a solvent to form a solution E. the temperature is 0 Karrow_forward(11.4, 11.7)In the Lewis structure of the molecule XY4, all X and Y bonds are single bonds and central atom X has one nonbonding pair (lone pair) of electrons. Predict the amolecular shape (geometry) of XY4 and b)hybridization of central atom (X). O a) tetrahedral b) sp³ O a) seesaw b) sp³d O a) tetrahedral b) sp³d² O a) trigonal bipyramidal b) sp³d²arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY