
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Question 5
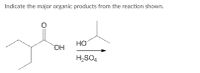
Transcribed Image Text:**Indicate the Major Organic Products from the Reaction Shown**
This image illustrates a chemical reaction involving organic compounds. The reactant on the left is a beta-hydroxy ketone, more specifically a 3-hydroxybutan-2-one (acetoin). The chemical structure shows a ketone group (>C=O) bonded to a carbon that also has a hydroxyl group (-OH).
The reaction involves the addition of an alcohol (on the right), specifically isopropanol (CH₃CHOHCH₃), in the presence of sulfuric acid (H₂SO₄) as a catalyst.
### Reaction Diagram:
- **Reactant:**
- Structure: The molecule has four carbon atoms. The second carbon (from the left) is double-bonded to an oxygen atom, and the third carbon is bonded to a hydroxyl group.
- **Catalyst:**
- H₂SO₄ (sulfuric acid) is indicated below the arrow, denoting its role in facilitating the reaction.
### Reaction Process:
This type of reaction typically involves acid-catalyzed dehydration or esterification, where the hydroxyl group may participate in forming an ether or an ester, depending on conditions.
### Expected Products:
In an acid-catalyzed reaction with an alcohol, potential products could involve:
1. **Ester Formation:** A possibility of forming an ester if the hydroxyl group reacts with the alcohol.
2. **Ether Formation:** Potential formation of an ether through the joining of the alcohol and ketone group.
The specific major organic product will depend on the reaction conditions and mechanism, which often require detailed analysis at the molecular level.
*Note: This explanation assumes a basic understanding of organic chemistry nomenclature and reaction types. It provides a general overview relevant for educational purposes.*
Expert Solution
arrow_forward
Step 1
Acid and alcohol react each other in presence of the catalytic amount of H2SO4 to form an ester as the major product along with water.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2-86 When each of the following measurements are length is convert to inches, using a conversion factor obtained from the equality 1 foot = figures should the answer have?, 12 inches, how many significantarrow_forward10 11 12 13 14 15 16 17 18 19 The particles in a gas vibrate faster than the particles in a liquid. O True O False 5.arrow_forward» Significant Figure Rules 01 >> 02 >> 03 >> 04 >> 05 >> All nonzero digits are significant. Zeros that appear between other nonzero digits are always significant. Zeros that appear in front of all of the nonzero digits are called left-end zeros. Left-end zeros are never significant. Zeros that appear after all nonzero digits are called right-end zeros. Right-end zeros in a number that locks a decimal point are not significant. ▬▬▬ 美銀銀時 ☐☐ Right-end zeros in a number with a decimal point are significant. This is true whether the zeros occur before or ofter the decimal point. 237 has three significant figures. 1.897 has four significant figures. 39,004 has five significant figures. 5.02 has three significant figures. 0.008 has one significant figure. 0.000416 has three significant figures. 140 has two significant figures. 75,210 has four significant figures. 620.0 has four significant figures. 19.000 has five significant figures. For multiplication and division problems, the answer…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY